16
Overview of Metabolism & the Provision of Metabolic Fuels
David A. Bender, PhD & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Explain what is meant by anabolic, catabolic and amphibolic metabolic pathways.
Explain what is meant by anabolic, catabolic and amphibolic metabolic pathways.
![]() Describe in outline the metabolism of carbohydrates, lipids and amino acids at the level of tissues and organs, and at the subcellular level, and how metabolic fuels are interconvertible.
Describe in outline the metabolism of carbohydrates, lipids and amino acids at the level of tissues and organs, and at the subcellular level, and how metabolic fuels are interconvertible.
![]() Describe the ways in which flux of metabolites through metabolic pathways is regulated.
Describe the ways in which flux of metabolites through metabolic pathways is regulated.
![]() Describe how a supple of metabolic fuels is provided in both the fed and fasting states; the formation of metabolic fuels reserves in the fed state and their mobilization in fasting.
Describe how a supple of metabolic fuels is provided in both the fed and fasting states; the formation of metabolic fuels reserves in the fed state and their mobilization in fasting.
BIOMEDICAL IMPORTANCE
Metabolism is the term used to describe the interconversion of chemical compounds in the body, the pathways taken by individual molecules, their interrelationships, and the mechanisms that regulate the flow of metabolites through the pathways. Metabolic pathways fall into three categories. (1) Anabolic pathways, which are those involved in the synthesis of larger and more complex compounds from smaller precursors—for example, the synthesis of protein from amino acids and the synthesis of reserves of triacylglycerol and glycogen. Anabolic pathways are endothermic. (2) Catabolic pathways, which are involved in the breakdown of larger molecules, commonly involving oxidative reactions; they are exothermic, producing reducing equivalents, and, mainly via the respiratory chain, ATP. (3) Amphibolic pathways, which occur at the “crossroads” of metabolism, acting as links between the anabolic and catabolic pathways, for example, the citric acid cycle.
Knowledge of normal metabolism is essential for an understanding of abnormalities underlying disease. Normal metabolism includes adaptation to periods of starvation, exercise, pregnancy, and lactation. Abnormal metabolism may result from nutritional deficiency, enzyme deficiency, abnormal secretion of hormones, or the actions of drugs and toxins.
A 70-kg adult human being requires about 8-12 MJ (1920-2900 kcal) from metabolic fuels each day, depending on the physical activity. Larger animals require less, and smaller animals more, per kg body weight, and growing children and animals have a proportionally higher requirement to allow for the energy cost of growth. For human beings, this requirement is met from carbohydrates (40-60%), lipids (mainly triacylglycerol, 30-40%), and protein (10-15%), as well as alcohol. The mix of carbohydrate, lipid, and protein being oxidized varies, depending on whether the subject is in the fed or fasting state, and on the duration and intensity of physical work.
The requirement for metabolic fuels is relatively constant throughout the day, since the average physical activity increases metabolic rate only by about 40-50% over the basal or resting metabolic rate. However, most people consume their daily intake of metabolic fuels in two or three meals, so there is a need to form reserves of carbohydrate (glycogen in liver and muscle) and lipid (triacylglycerol in adipose tissue) in the period following a meal, for use during the intervening time when there is no intake of food.
If the intake of metabolic fuels is consistently greater than energy expenditure, the surplus is stored, largely as triacylglycerol in adipose tissue, leading to the development of obesity and its associated health hazards. By contrast, if the intake of metabolic fuels is consistently lower than energy expenditure, there are negligible reserves of fat and carbohydrate, and amino acids arising from protein turnover are used for energy-yielding metabolism rather than replacement protein synthesis, leading to emaciation, wasting, and, eventually, death (see Chapter 43).
In the fed state, after a meal, there is an ample supply of carbohydrate, and the metabolic fuel for most tissues is glucose. In the fasting state glucose must be spared for use by the central nervous system (which is largely dependent on glucose) and the red blood cells (which are wholly reliant on glucose). Therefore, tissues that can use fuels other than glucose do so; muscle and liver oxidize fatty acids and the liver synthesizes ketone bodies from fatty acids to export to muscle and other tissues. As glycogen reserves become depleted, amino acids arising from protein turnover are used for gluconeogenesis.
The formation and utilization of reserves of triacylglycerol and glycogen, and the extent to which tissues take up and oxidize glucose, are largely controlled by the hormones insulin and glucagon. In diabetes mellitus, there is either impaired synthesis and secretion of insulin (type I diabetes, sometimes called juvenile onset, or insulin-dependent diabetes) or impaired sensitivity of tissues to insulin action (type II diabetes, sometimes called adult onset or noninsulin-dependent diabetes), leading to severe metabolic derangement. In cattle, the demands of heavy lactation can lead to ketosis, as can the demands of twin pregnancy in sheep.
PATHWAYS THAT PROCESS THE MAJOR PRODUCTS OF DIGESTION
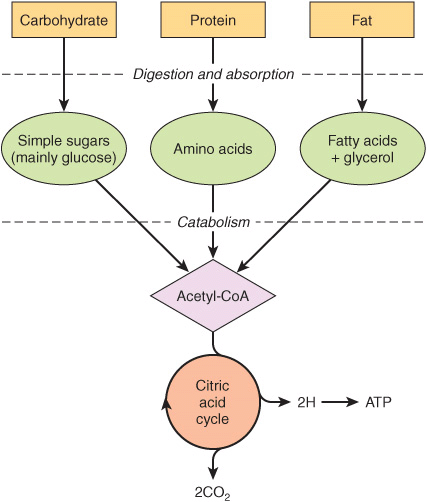
The nature of the diet sets the basic pattern of metabolism. There is a need to process the products of digestion of dietary carbohydrate, lipid, and protein. These are mainly glucose, fatty acids and glycerol, and amino acids, respectively. In ruminants (and, to a lesser extent, other herbivores), dietary cellulose is fermented by symbiotic microorganisms to short-chain fatty acids (acetic, propionic, butyric), and metabolism in these animals is adapted to use these fatty acids as major substrates. All the products of digestion are metabolized to a common product, acetyl-CoA, which is then oxidized by the citric acid cycle (Figure 16–1).
FIGURE 16–1 Outline of the pathways for the catabolism of dietary carbohydrate, protein, and fat. All the pathways lead to the production of acetyl-CoA, which is oxidized in the citric acid cycle, ultimately yielding ATP by the process of oxidative phosphorylation.
Carbohydrate Metabolism Is Centered on the Provision & Fate of Glucose
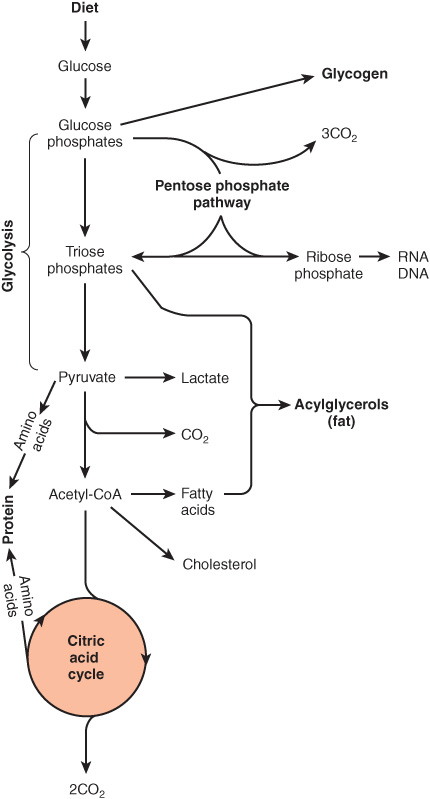
Glucose is the major fuel of most tissues (Figure 16–2). It is metabolized to pyruvate by the pathway of glycolysis. Aerobic tissues metabolize pyruvate to acetyl-CoA, which can enter the citric acid cycle for complete oxidation to CO2 and H2O, linked to the formation of ATP in the process of oxidative phosphorylation (Figure 13–2). Glycolysis can also occur anaerobically (in the absence of oxygen) when the end product is lactate.
FIGURE 16–2 Overview of carbohydrate metabolism showing the major pathways and end products. Gluconeogenesis is not shown.
Glucose and its metabolites also take part in other processes, eg. (1) Synthesis of the storage polymer glycogen in skeletal muscle and liver. (2) The pentose phosphate pathway, an alternative to part of the pathway of glycolysis. It is a source of reducing equivalents (NADPH) for fatty acid synthesis and the source of ribose for nucleotide and nucleic acid synthesis. (3) Triose phosphates give rise to the glycerol moiety of triacylglycerols. (4) Pyruvate and intermediates of the citric acid cycle provide the carbon skeletons for the synthesis of nonessential or dispensable amino acids, and acetyl-CoA is the precursor of fatty acids and cholesterol (and hence of all steroids synthesized in the body). Gluconeogenesis is the process of forming glucose from noncarbohydrate precursors, for example, lactate, amino acids, and glycerol.
Lipid Metabolism Is Concerned Mainly with Fatty Acids & Cholesterol
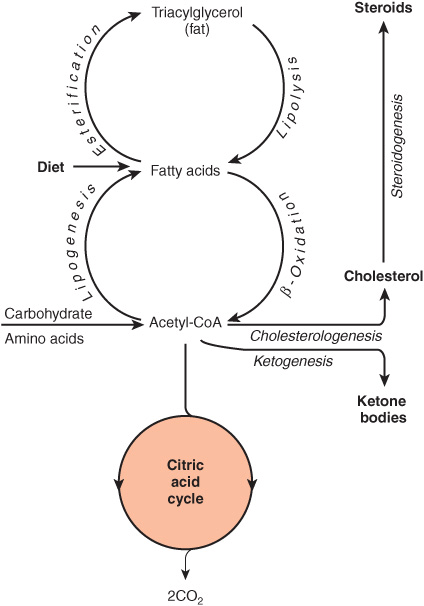
The source of long-chain fatty acids is either dietary lipid or de novo synthesis from acetyl-CoA derived from carbohydrate or amino acids. Fatty acids may be oxidized to acetyl-CoA (β-oxidation) or esterified with glycerol, forming triacylglycerol (fat) as the body’s main fuel reserve.
Acetyl-CoA formed by β-oxidation may undergo three fates (Figure 16–3).
FIGURE 1–3 Overview of fatty acid metabolism showing the major pathways and end products. The ketone bodies are acetoacetate, 3-hydroxybutyrate, and acetone.
1. As with acetyl-CoA arising from glycolysis, it is oxidized to CO2 + H2O via the citric acid cycle.
2. It is the precursor for synthesis of cholesterol and other steroids.
3. In the liver, it is used to form ketone bodies (acetoacetate and 3-hydroxybutyrate), which are important fuels in prolonged fasting and starvation.
Much of Amino Acid Metabolism Involves Transamination
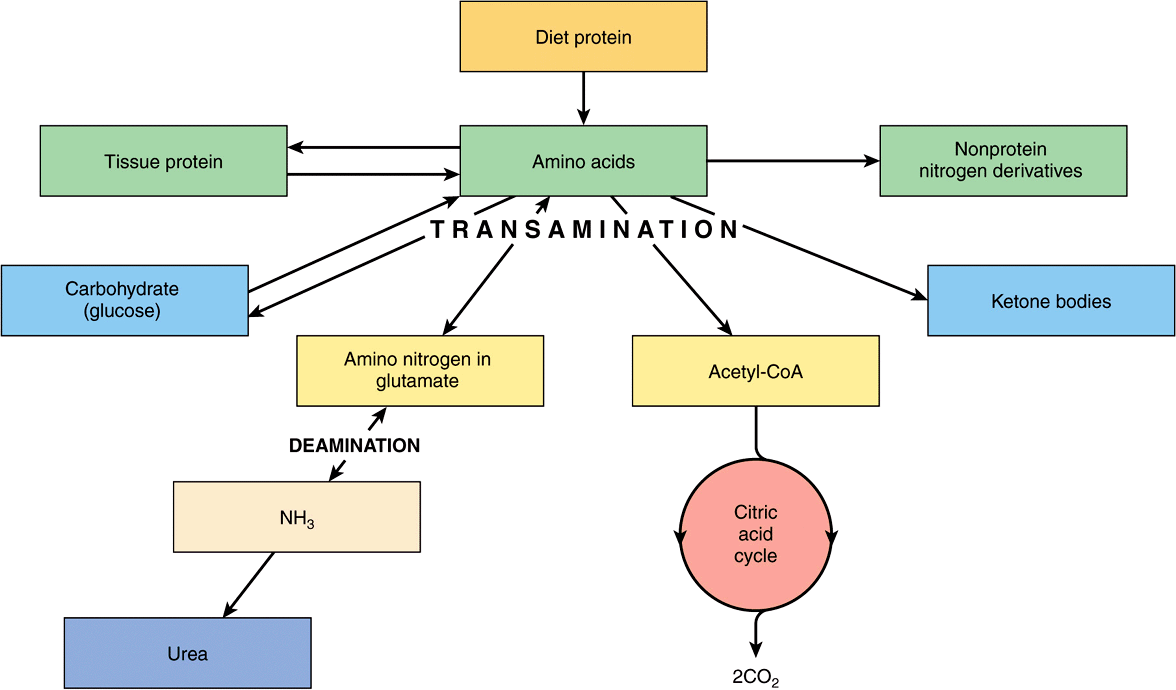
The amino acids are required for protein synthesis (Figure 16–4). Some must be supplied in the diet (the essential or indispensable amino acids), since they cannot be synthesized in the body. The remainder are nonessential or dispensable amino acids, which are supplied in the diet, but can also be formed from metabolic intermediates by transamination using the amino group from other amino acids. After deamination, amino nitrogen is excreted as urea, and the carbon skeletons that remain after transamination may (1) be oxidized to CO2 via the citric acid cycle, (2) be used to synthesize glucose (gluconeogenesis), or (3) form ketone bodies or acetyl CoA, which may be oxidized or be used for synthesis of fatty acids.
FIGURE 16–4 Overview of amino acid metabolism showing the major pathways and end products.
Several amino acids are also the precursors of other compounds, for example, purines, pyrimidines, hormones such as epinephrine and thyroxine, and neurotransmitters.
METABOLIC PATHWAYS MAY BE STUDIED AT DIFFERENT LEVELS OF ORGANIZATION
In addition to studies in the whole organism, the location and integration of metabolic pathways is revealed by studies at several levels of organization. (1) At the tissue and organ level the nature of the substrates entering and metabolites leaving tissues and organs is defined. (2) At the subcellular level each cell organelle (eg, the mitochondrion) or compartment (eg, the cytosol) has specific roles that form part of a subcellular pattern of metabolic pathways.
At the Tissue & Organ Level, the Blood Circulation Integrates Metabolism
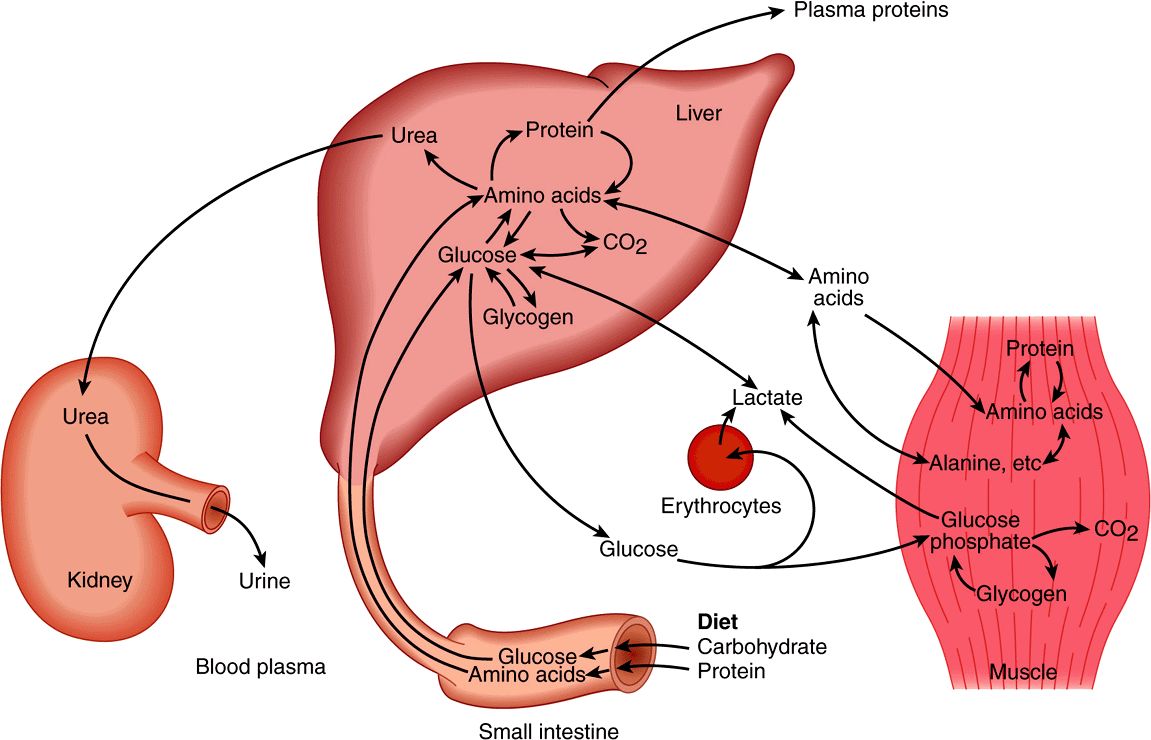
Amino acids resulting from the digestion of dietary protein and glucose resulting from the digestion of carbohydrates are absorbed via the hepatic portal vein. The liver has the role of regulating the blood concentration of these water-soluble metabolites (Figure 16–5). In the case of glucose, this is achieved by taking up glucose in excess of immediate requirements and using it to synthesize glycogen (glycogenesis, Chapter 19) or to fatty acids (lipogenesis, Chapter 23). Between meals, the liver acts to maintain the blood glucose concentration by breaking down glycogen (glycogenolysis, Chapter 19) and, together with the kidney, by converting non-carbohydrate metabolites such as lactate, glycerol, and amino acids to glucose (gluconeogenesis, Chapter 20). The maintenance of an adequate concentration of blood glucose is vital for those tissues for which it is the major fuel (the brain) or the only fuel (erythrocytes). The liver also synthesizes the major plasma proteins (eg, albumin) and deaminates amino acids that are in excess of requirements, synthesizing urea, which is transported to the kidney and excreted (Chapter 28).
FIGURE 16–5 Transport and fate of major carbohydrate and amino acid substrates and metabolites. Note that there is little free glucose in muscle, since it is rapidly phosphorylated upon entry.
Skeletal muscle utilizes glucose as a fuel, both aerobically, forming CO2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree