45
Free Radicals and Antioxidant Nutrients
OBJECTIVES
After studying this chapter, you should be able to:
![]() Describe the damage caused to DNA, lipids, and proteins by free radicals, and the diseases associated with radical damage.
Describe the damage caused to DNA, lipids, and proteins by free radicals, and the diseases associated with radical damage.
![]() Describe the main sources of oxygen radicals in the body.
Describe the main sources of oxygen radicals in the body.
![]() Describe the mechanisms and dietary factors that protect against radical damage.
Describe the mechanisms and dietary factors that protect against radical damage.
![]() Explain how antioxidants can also act as pro-oxidants, and why intervention trials of antioxidant nutrients have generally yielded disappointing results.
Explain how antioxidants can also act as pro-oxidants, and why intervention trials of antioxidant nutrients have generally yielded disappointing results.
BIOMEDICAL IMPORTANCE
Free radicals are formed in the body under normal conditions. They cause damage to nucleic acids, proteins, and lipids in cell membranes and plasma lipoproteins. This can cause cancer, atherosclerosis and coronary artery disease, and autoimmune diseases. Epidemiological and laboratory studies have identified a number of protective antioxidant nutrients: selenium, vitamins C and E, β-carotene, and other carotenoids, and a variety of polyphenolic compounds derived from plant foods. Many people take supplements of one or more antioxidant nutrients. However, intervention trials show little benefit of antioxidant supplements except among people who were initially deficient, and many trials of β-carotene and vitamin E have shown increased mortality among those taking the supplements.
Free Radical Reactions Are Self-Perpetuating Chain Reactions
Free radicals are highly reactive molecular species with an unpaired electron; they persist for only a very short time (of the order of 10-9 to 10-12 sec) before they collide with another molecule and either abstract or donate an electron in order to achieve stability. In so doing, they generate a new radical from the molecule with which they collided. The main way in which a free radical can be quenched, so terminating this chain reaction, is if two radicals react together, when the unpaired electrons can become paired in one or other of the parent molecules. This is a rare occurrence, because of the very short half-life of an individual radical and the very low concentrations of radicals in tissues.
The most damaging radicals in biological systems are oxygen radicals (sometimes called reactive oxygen species)—especially superoxide, ![]() , hydroxyl, OH•, and perhydroxyl, O2H• Tissue damage caused by oxygen radicals is often called oxidative damage, and factors that protect against oxygen radical damage are known as antioxidants.
, hydroxyl, OH•, and perhydroxyl, O2H• Tissue damage caused by oxygen radicals is often called oxidative damage, and factors that protect against oxygen radical damage are known as antioxidants.
Radicals Can Damage DNA, Lipids, and Proteins
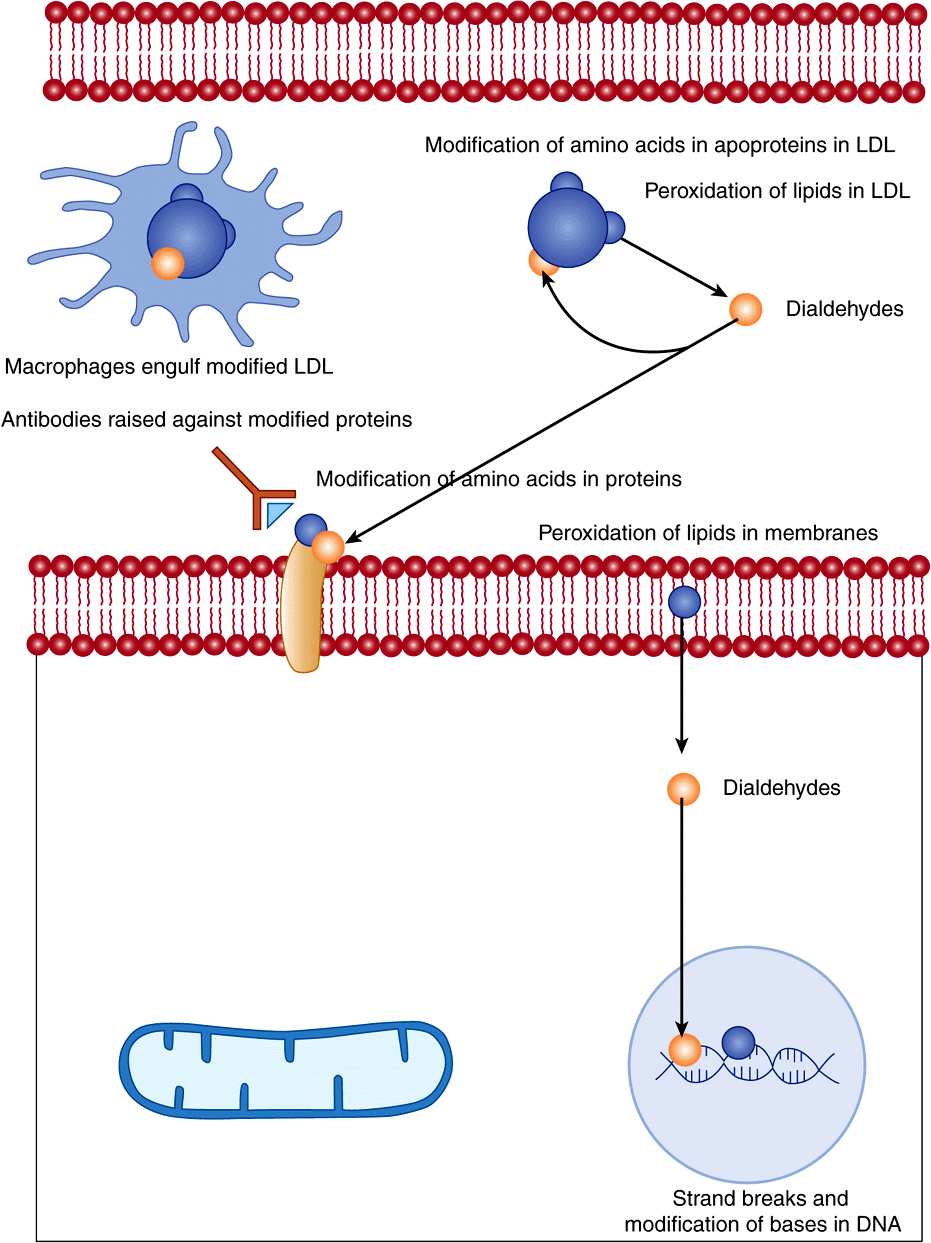
Interaction of radicals with bases in DNA can lead to chemical changes that, if not repaired (Chapter 35), may be inherited in daughter cells. Radical damage to unsaturated fatty acids in cell membranes and plasma lipoproteins leads to the formation of lipid peroxides, then highly reactive dialdehydes that can chemically modify proteins and nucleic acid bases. Proteins are also subject to direct chemical modification by interaction with radicals. Oxidative damage to tyrosine residues in proteins can lead to the formation of dihydroxyphenylalanine that can undergo nonenzymic reactions leading to further formation of oxygen radicals (Figure 45–1).
FIGURE 45–1 Tissue damage by radicals.
The total body radical burden can be estimated by measuring the products of lipid peroxidation. Lipid peroxides can be measured by the ferrous oxidation in xylenol orange (FOX) assay. Under acidic conditions, they oxidize Fe2+ to Fe3+, which forms a chromophore with xylenol orange. The dialdehydes formed from lipid peroxides can be measured by reaction with thiobarbituric acid, when they form a red fluorescent adduct—the results of this assay are generally reported as total thiobarbituric acid reactive substances, TBARS. Peroxidation of n-6 polyunsaturated fatty acids leads to the formation of pentane, and of n-3 polyunsaturated fatty acids to ethane, both of which can be measured in exhaled air.
Radical Damage May Cause Mutations, Cancer, Autoimmune Disease, and Atherosclerosis
Radical damage to DNA in germline cells in ovaries and testes can lead to heritable mutations; in somatic cells, the result may be initiation of cancer. The dialdehydes formed as a result of radical-induced lipid peroxidation in cell membranes can also modify bases in DNA.
Chemical modification of amino acids in proteins, either by direct radical action or as a result of reaction with the products of radical-induced lipid peroxidation, leads to proteins that are recognized as nonself by the immune system. The resultant antibodies will also cross-react with normal tissue proteins, so initiating autoimmune disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree