Overview of Factors Affecting Drug Discovery
Laws of Project Management
Murphy’s Law: If anything can go wrong, it will.
O’Toole’s Commentary on Murphy’s Law: Murphy was an optimist.
Nonreciprocal Laws of Expectations: Negative expectations yield negative results. Positive expectations yield negative results.
Howe’s Law: Every man has a scheme that will not work.
Zymurgy’s First Law of Evolving System Dynamics: Once you open a can of worms, the only way to recan them is to use a larger can, giving you a bigger can of worms.
6. Gordon’s First Law: If a research project is not worth doing at all, it is not worth doing well.
Maler’s Law: If the facts do not confirm the theory, they must be disposed of.
Boren’s First Law of Communication: When in doubt, mumble.
Ninety-Ninety Rule of Project Scheduling: The first 90% of the job takes 90% of the time, and the last 10% takes the other 90%.
Law of Project Arithmetic: Some of it plus the rest of it equals all of it.
THE MATRIX APPROACH AS A FRAME OF REFERENCE
The numerous factors that influence innovation may be viewed from many perspectives. The perspective described in this chapter focuses on a matrix model composed of levels of organization, categories of influence, and types of organization, as depicted in Fig. 7.1.
The matrix approach is a useful frame of reference to view both major and minor factors that affect and influence innovation. Major categories of influence include various economic, social, regulatory, medical practice, and scientific factors. The discussion focuses on how these five major categories operate within the four major levels of organization in the matrix: national/international, institutional, departmental, and individual. The major types of organizations at which innovation occurs form the third arm of this matrix: academic, government, pharmaceutical industry, and others, such as professional societies or professional associations.
This matrix has 80 individual cubes that could be discussed, although not every one would be worth investigating and describing, and some are much more interesting than others. The 80 individual cubes should be thought of as encompassing both the macro and the micro levels of factors that affect innovation.
The remainder of this discussion focuses on how the major categories of influence operate within each of the four major levels of organization—national, institutional, departmental, and individual.
NATIONAL LEVEL
At the national (macro) level, there is clearly an overlap among the five major categories that influence drug discoveries: economics, regulatory policy, social policy, medical practice, and the state of the art in science.
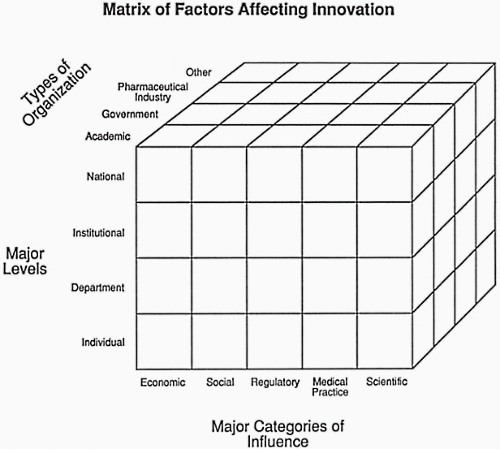 Figure 7.1 Matrix of factors affecting innovation based on types of organization, organizational levels, and types of influence or pressure. |
Economic Policies
The economic policies of a country influence innovation through many practices, but only three are mentioned: tax laws, patent laws, and pricing policies. Tax laws provide incentives (or disincentives) for various groups to invest in drug discovery. If tax write-offs for research activities were made looser or tighter, it is certain that this would have a stimulating or inhibiting effect on innovation. Patents are probably the single most important factor that influences innovation on the national level, as well as on the institutional and departmental levels. Over the past two decades, a lot of attention has been paid, at least in the United States and more and more elsewhere, to patent laws. It has been said that, during the late 1970s and early 1980s, the Pharmaceutical Manufacturers Association now known as the Pharmaceutical Research and Manufacturers of America focused most of its efforts on attempts to revise the patent laws. The Pharmaceutical Manufacturers Association helped achieve the Patent-Term Restoration Act of 1984 in the United States.
National governmental authorities control prices for new drugs in most countries. The policies these agencies adopt and use have a major influence on the willingness of some groups to invest in innovation and discovery. The pricing of drugs has not had a great impact on innovation in the past but probably will have increased impact in the future. Patent laws and pricing policies may be viewed from both an economic and a regulatory perspective.
Regulatory Policies
Other regulatory influences on innovation (apart from patent laws and pricing policies) come from certain national authorities, such as the Department of Health in the United Kingdom and the Food and Drug Administration in the United States, which must approve new drugs for marketing. Companies have been known to avoid the search for new drugs in specific disease areas because of a belief that an important regulatory agency was unlikely to approve for marketing any such drugs that the company might discover. For example, several years ago, a drug to treat cocaine abuse would usually not have been pursued by a pharmaceutical company because of the regulatory concern about the potential for abusing the new drug. Part of the reasoning for this belief is based on the personalities of important decision-making regulators and their opinions. Contraceptive research has had periods where it blossomed and dry periods, which were influenced in part by regulatory attitudes as well as by science.
Social Policies
Social policies are dependent on the type of organization that people work in, the ethical standards followed by the organization, and the public image and reputation of scientists, physicians, and others who are engaged in drug discovery. This image is primarily influenced by the media, as well as by the scientists’ own efforts, contacts, and achievements. A positive image encourages many of the most creative people in society to choose scientific careers.
Fewer students in recent years have been choosing careers in the sciences. This may be partly a result of the many stories that discuss fraud, deception, greed, and misconduct that have somewhat tarnished the public image of scientists and the pharmaceutical industry. The issue of fraud in science is being addressed in numerous positive ways by journal editors and university committees. Universities and other groups are establishing standards and guidelines designed to self-police the integrity of research and reduce the likelihood of fraud. Scientists must accept responsibility for the work that they publish, and journals are now forcing them to include as authors only colleagues who have made a substantial contribution to the project. In many ways, the whole issue of fraud is being dealt with better than the issue of deception. Deception is not spoken about as much as fraud, but it exists in many forms (e.g., in clinical trials where patients were sometimes deceived by not being told that they may receive a placebo or by being told that they would receive a placebo when they were being given an active drug).
Medical Practice
The diagnosis of many medical problems as specific diseases or syndromes is highly variable among countries. As a result, the diseases that “exist” differ from country to country. Some of these differences are real, others are artificial, and a third group is hard to characterize. There are numerous diseases that are relatively common in some countries but that are not believed to exist in others. In Germany, for example, low blood pressure is frequently diagnosed as the disease hypotension, yet this disease is not believed to exist in many other countries. German physicians treat hypotensive patients with drugs to raise their blood pressure. On the other hand, German physicians do not diagnose irritable bowel syndrome, which is a relatively common diagnosis in many other countries. Few German researchers and pharmaceutical companies would try to discover treatments for irritable bowel syndrome, although a new compound for this disease could be sought for eventual testing in another country. It is less likely that original research would be conducted on a disease that physicians do not believe exists.
The culture of a country and the diseases that they consider particularly important influence the allocation of funds for innovation research. In general, French physicians emphasize the liver to a larger extent than do physicians in other countries and diagnose and treat many medical problems as if they are caused by liver abnormalities (Payer 1988). The French also discuss more diseases that are said to influence the liver than do other nationalities. In Germany, the heart is often considered to be of paramount importance, and many diseases are looked at in terms of how they influence or are influenced by the heart, which is quite differently from the way they are viewed in other countries (Payer 1988). Hyperkinetic children are seen to be more of a problem in the United States than in the United Kingdom, where many professionals believe Americans are spoiling their children and overdiagnosing this problem. Therefore, few companies in the United Kingdom would be expected to invest significant funds to look for new drugs in that particular disease area.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


