Ovarian Mucinous Tumors
General Features
Mucinous tumors show cysts and glands lined by epithelial cells containing intracytoplasmic mucin. The tumor cells may resemble those of the endocervix, gastric pylorus, or intestine. They are typically diastase resistant PAS positive, and mucicarmine positive. Mucinous tumors account for 10–15% of all primary ovarian tumors.1 Approximately 80% are benign and the remainder are borderline tumors, noninvasive carcinomas, and invasive carcinomas.1 Although they generally occur in older women (mean ages 51–54 years), mucinous borderline tumors and carcinomas are more common in the first two decades than their serous counterparts.2 Mucinous tumors, especially the borderline tumors, tend to be the largest of all ovarian tumors. Many of them are 15–30 cm in diameter and weigh up to 4000 g or more.2
Although mucinous ovarian tumors are currently classified as surface epithelial tumors, their origin is unclear in most cases. Some mucinous tumors are of germ cell origin (monodermal teratomas), but neometaplasia of the ovarian surface epithelium is an alternative explanation for their development.2 In fact, transitions between mucinous tumors and serous and endometrioid tumors are occasionally seen. Also, serous and endometrioid tumors may secrete mucin from the apical pole of the epithelial cells, resulting in abundant intracystic mucus, but are not generally intracytoplasmic. Mucinous tumors may be associated with dermoid cysts (3–5%), Brenner tumors, and mucinous tumors of other organs such as the uterine cervix and appendix.2 In patients with the Peutz–Jeghers syndrome, well-differentiated mucinous ovarian tumors may be accompanied by minimal deviation adenocarcinomas (‘adenoma malignum’) of the cervix. Occasionally, mucinous cystic ovarian tumors may develop from heterologous gastrointestinal elements in a Sertoli–Leydig cell tumor.
Mucinous ovarian tumors are among the most common non-endocrine neoplasms presenting hormonal manifestations. Most times, the endocrine symptoms are due to the secretion of steroid hormones, which the ovarian stroma adjacent to the tumor produces.2,3 The serum level of inhibin (a hormone produced by ovarian granulosa and lutein cells that inhibits the secretion of follicle-stimulating hormone by the anterior pituitary gland) is considered to be a tumor marker for mucinous borderline tumors and carcinomas, possibly due to the reactive luteinized cells that develop in the adjacent stroma.4 Less frequently, patients present with Zollinger–Ellison syndrome, secondary to gastrin secretion by neuroendocrine cells in the gastrointestinal mucinous epithelium, and rarely, the carcinoid syndrome (see Chapter 29). CA125, carcinoembryonic antigen (CEA), and CA19-9 are often elevated in mucinous carcinomas.
Ovarian mucinous tumors are subdivided into benign, borderline, and malignant categories depending on their degree of cell proliferation, nuclear atypia, and the presence or absence of stromal invasion. In contrast to serous tumors, which are characteristically homogeneous in their degree of differentiation, mucinous tumors often are heterogeneous. Benign-appearing, borderline, and invasive patterns may coexist within an individual neoplasm; also, not infrequently, the degree of malignancy of the carcinomatous component varies from noninvasive to invasive and from well-differentiated to poorly differentiated or even undifferentiated (anaplastic) carcinoma. Such morphologic continuum suggests that tumor progression occurs from cystadenoma and borderline tumor to noninvasive, microinvasive, and invasive carcinoma. This hypothesis is supported by studies of K-RAS mutations, which are common in mucinous ovarian tumors and represent an early event in mucinous ovarian tumorigenesis. Mucinous borderline tumors have a higher frequency of K-RAS mutations than that of mucinous cystadenomas, but lower than mucinous carcinomas.5–9 Using microdissection, the same K-RAS mutation has been detected in benign-appearing, borderline, and malignant areas of the same tumor.5 From a practical viewpoint, the prognostic evaluation of mucinous ovarian tumors other than benign cystadenomas is difficult because of their typical large size, and the great variation in the degree of differentiation of individual tumors. Extensive sampling is important, especially of areas that appear nodular or solid.
Benign Mucinous Tumors
Macroscopic Features
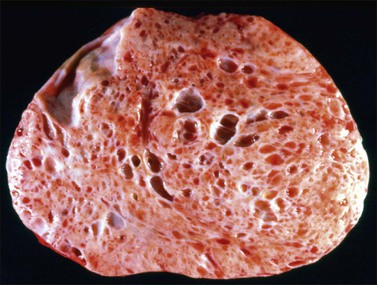
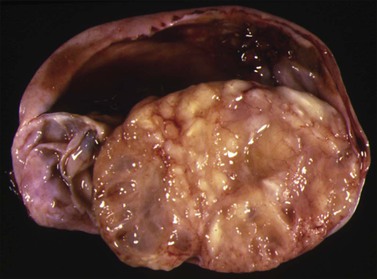
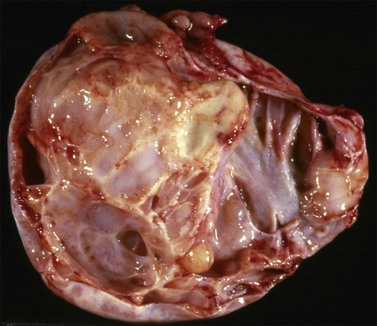
Mucinous cystadenomas are often large (mean size, 10 cm), unilateral, multilocular, but sometimes unilocular cystic tumors containing mucoid material. The outer cyst wall is often thick with a smooth or bosselated surface. The rare mucinous cystadenofibromas and adenofibromas are partly to almost completely solid with small cysts (Figure 26.1). Benign mucinous tumors are typically unilateral in 95% of cases.
Microscopic Features
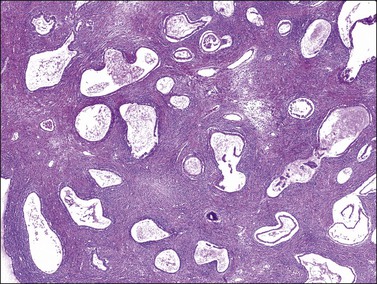
Mucinous cystadenomas are composed of glands and cysts lined by a single layer of columnar cells with abundant intracellular mucin. Cellular stratification is minimal, and nuclei are basally located with only mild atypia (Figure 26.2).10 If epithelial proliferation resembling a borderline mucinous tumor is present, this feature must be limited to <10% of the epithelial volume for the tumor to qualify as a cystadenoma.11 Papillae are unusual except in rare cases of mucinous cystadenomas of endocervical type, which are conspicuously papillary. Goblet cells, neuroendocrine cells, and, rarely, Paneth cells may be encountered in mucinous cystadenomas of gastrointestinal type. However, gastric foveolar-type epithelium or intestinal epithelium with goblet cells is found more often in mucinous borderline tumors and carcinomas. Reactive nuclear atypia and mitotic activity can be seen in peripheral crypt-like structures. The stroma is fibrocollagenous and exhibits variable cellularity. Occasionally, marked prominence of the stroma occurs between glands, giving rise to patterns of adenofibroma (Figure 26.3). Stromal cellularity may be increased in the vicinity of the epithelium where lutein-like cells are seen in 25% of cases. Rare benign tumors may show definite Leydig cells (with crystals) in the adjacent stroma. Smooth muscle may develop sometimes in the stroma running parallel to the cyst linings.
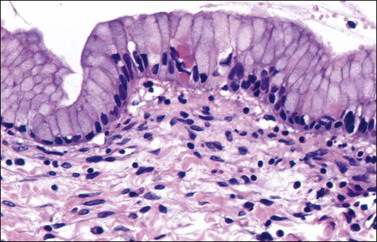
Figure 26.2 Mucinous cystadenoma. The cytoplasms appear full of mucin and the nuclei are small and basal.
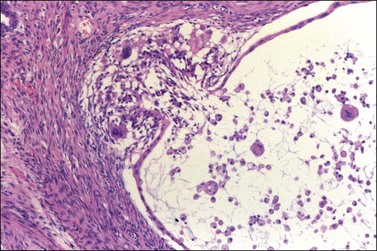
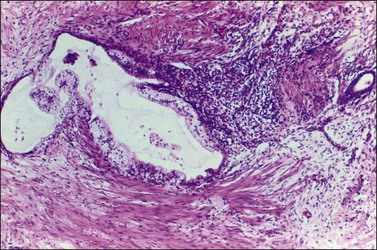
Rupture of mucinous glands and cysts is common, resulting in extravasation of mucin into the stroma, and often a marked inflammatory response. Such a finding must be distinguished from large pools of dissecting mucin (pseudomyxoma ovarii), which is a characteristic feature of mucinous ovarian tumors associated with pseudomyxoma peritonei (see later). In about 5% of mucinous cystadenomas, the mucin in the stroma typically elicits a histiocytic and foreign body giant cell response (mucin granuloma) (Figure 26.4). Extruded neoplastic epithelium from an adjacent ruptured gland, particularly if accompanied by a mucin granuloma, should be distinguished from stromal microinvasion (see later). Mucinous cystadenomas and adenofibromas are benign but can recur if incompletely excised. Tumor rupture is not associated with recurrence.
Mucinous cystadenomas are associated with dermoid cysts in 5% of cases2 and with Brenner tumors in 18%.12 Accordingly, it has been suggested that mucinous tumors of intestinal type may be of germ cell origin or arise from Brenner tumors.
Mucinous Borderline Tumors
Mucinous borderline tumors (MBTs) exhibit an epithelial proliferation of mucinous-type cells greater than that seen in their benign counterparts, but without destructive stromal invasion. The proliferative areas must constitute greater than 10% of the epithelial volume of the tumor.11 In the Western world, MBTs are less common than serous borderline tumors (SBTs). Of the 20% of primary mucinous tumors that are not cystadenomas, MBTs outnumber the invasive carcinomas.13 MBTs have been subclassified into two different clinicopathologic forms: the most common form is composed of gastrointestinal-type epithelium and is designated MBT of gastrointestinal type. A second and less common variant of MBT contains endocervical-type epithelium and has been named MBT endocervical-like (Figure 26.5).14
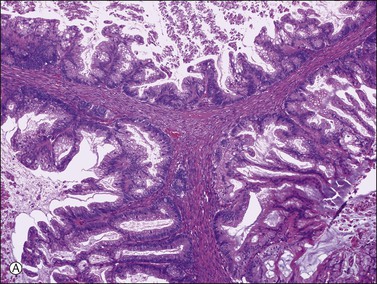
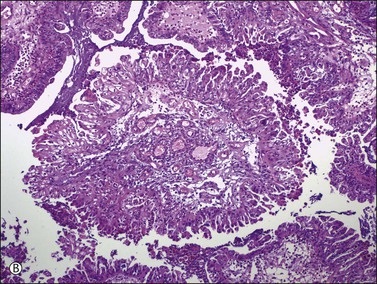
Figure 26.5 Mucinous borderline tumors. (A) The gastrointestinal-type tumor resembles a colonic polyp and contains goblet cells. (B) The endocervical-like (müllerian type) tumor shows mucinous epithelial cells resembling endocervical epithelium (upper left) and indifferent cells. None of the tumors shows stromal invasion.
Mucinous Borderline Tumors, Endocervical-Like
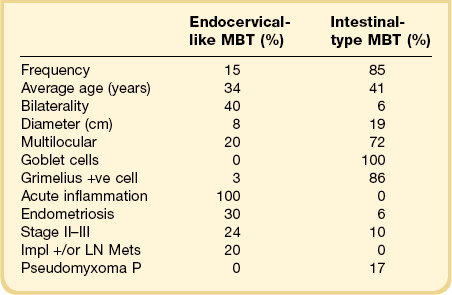
Endocervical MBTs,14 also designated as müllerian MBTs, account for 10–15% of MBTs. About 140 cases have been reported.14–17 These tumors differ in many respects from intestinal MBTs, as shown in Table 26.1. The average age of the patients with endocervical MBTs is 40 years, with a range of 15–84 years.14–17 An association with endometriosis is frequent (35–50%).16 At the time of diagnosis, most tumors are confined to the ovary and approximately 20% have spread to the peritoneum or regional lymph nodes.14 No association with pseudomyxoma peritonei has been described.
Macroscopic Features
The tumors average 8–10 cm in diameter, with a range from 2 to 36 cm. About 80% are unilocular or contain three or fewer locules. Most of them show grossly visible intracystic papillae (Figure 26.6). From 12% to 40% are bilateral at presentation14–17 and the contralateral ovary is subsequently involved by an endocervical-type MBT in an additional 7% of cases.14
Microscopic Features
The tumors contain complex papillae architecturally similar to those of SBTs. The epithelial lining is composed of columnar, mucin-containing cells that resemble endocervical cells as well as indifferent polygonal cells with abundant eosinophilic cytoplasm that are usually located at the tips of papillae (Figure 26.7).14 The nuclei exhibit mild to focally severe atypia. Mitotic figures are infrequent. No destructive invasion of the stroma is observed. Nuclear stratification in the absence of recognizable stromal invasion cannot be used as a diagnostic criterion of carcinoma in endocervical-type MBTs as the polygonal eosinophilic cells may be stratified up to 20 or more in height (Figure 26.8).14 Rare tumors exhibit foci of micropapillary growth similar to the micropapillary pattern seen in SBTs.15 Other epithelial cells of müllerian type may be present, including ciliated serous cells, endometrioid cells, and squamous epithelium.18,19 From 30% to 50% of the tumors show a transition from mucinous neoplasia to endometriosis.14,16,18 No areas of intestinal differentiation are usually seen. Typically, polymorphonuclear leukocytes infiltrate the stroma of the papillae, the neoplastic epithelium, and the intraluminal mucin in almost all cases (Figure 26.9).
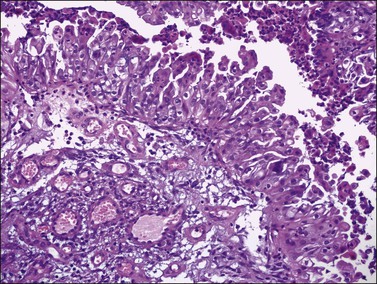
Figure 26.7 Mucinous borderline tumor, endocervical-like (müllerian type). Mucinous and indifferent cells with eosinophilic cytoplasm and tufting. A neutrophil infiltration is seen.
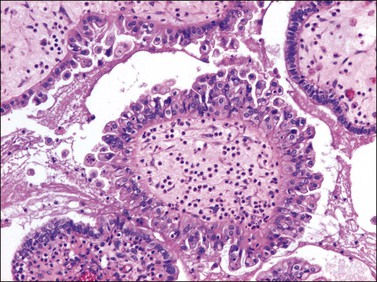
Figure 26.8 Mucinous borderline tumor, endocervical-like (müllerian type). Stratification with cellular budding is evident. Neutrophils are seen in the stroma and epithelium. (Reproduced with permission of Lippincott Williams & Wilkins.)
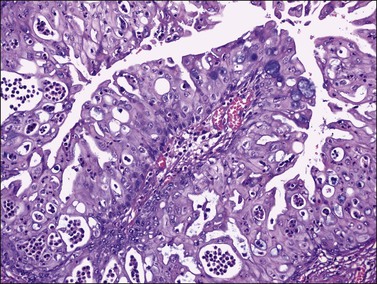
Figure 26.9 Mucinous borderline tumor, endocervical-like (müllerian type). Cellular stratification and prominent intraepithelial neutrophil infiltration.
Endocervical-type MBTs with intraepithelial carcinoma have been described.15–17 Morphologic features of the intraepithelial carcinoma include foci showing a cribriform pattern of growth and stroma-free cellular papillae, or nuclear features of malignancy (Figure 26.10). In most cases, the intraepithelial carcinoma in endocervical-type MBT consists of multiple foci usually measuring less than 1 or 2 mm in linear dimension. Classification of these tumors as intraepithelial carcinoma seems justified by the mean 7 year disease-free follow-up interval for 10 patients with this diagnosis.15–17 Since few cases have been reported and the follow-up intervals were relatively short, the favorable behavior of these tumors needs confirmation by additional investigations.
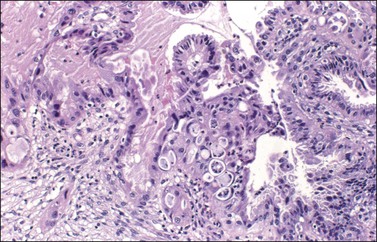
Figure 26.10 Mucinous endocervical-like borderline tumor with IEC and microinvasion. The lining epithelium shows cell stratification, cribriform pattern, and marked nuclear atypia. A nest of invasive cells is seen at the lower left corner.
Microinvasion has been described in 24 endocervical-type MBTs (19%) from five different series.15–17,20,21 The microinvasive foci ranged from <2 to 5 mm in greatest diameter, and were similar to those found more commonly in SBTs (Figure 26.10). Twelve patients with prolonged follow-up were without evidence of disease at an average of 8 years.15–17,20,21
Endocervical-type MBTs may be associated with pelvic or abdominal implants (3–20%), which are characteristically discrete and contain mucinous glands in a fibrous stroma (desmoplastic implants) (Figure 26.11).14,16 In some cases the peritoneal implants may arise from independent foci of endometriosis. Some implants may appear invasive.
Immunohistochemistry and Somatic Genetics
Endocervical MBTs frequently express cytokeratin 7 (CK7; 100%), estrogen receptor (ER; 100%), progesterone receptor (PR; 67%), CA125 (92%), and mesothelin (83%). Expression of WT1 is rare (8%) and, in contrast to gastrointestinal MBTs, immunoreactivity for CK20 and CDX2 is absent.22–24 Therefore, the gastrointestinal and endocervical types of MBTs are immunophenotypically distinctive tumors. Whereas the former express markers of intestinal-type differentiation (CK20 and CDX2), the latter show expression of müllerian-type markers (ER, PR, CA125, and mesothelin). The müllerian immunoprofile of endocervical MBTs, as well as their frequent association with endometriosis, supports the concept that this subtype of mucinous tumors is closely related to endometrioid tumors and justifies the designation ‘müllerian.’ Designating this tumor partially as ‘seromucinous’ is incomplete, and ignores the common presence of other müllerian components such as endometrioid or squamous elements.19 Furthermore, the recent finding in these tumors of loss of expression or mutation of ARID1A, which occurs in most endometriosis-associated ovarian tumors but not in pure serous tumors, favors the term müllerian over seromucinous.25
Treatment and Prognosis
Stage I endocervical-type MBTs are generally treated like SBTs (see Chapter 25). If the contralateral normal-appearing ovary is conserved, the patient should be followed closely for possible development of a similar tumor in it. The prognosis of endocervical-type MBT is excellent and approximates that of SBTs. Recent studies report that foci of IEC or microinvasion do not influence the prognosis.15–17 No deaths from these tumors have been well documented, although most reported follow-up has been under 5 years.14–16,26–28 There is no evidence that chemotherapy is necessary or helpful, even for patients with higher stage disease.2,14
Mucinous Borderline Tumors, Gastrointestinal Type
These tumors account for 85% of MBTs and occur most frequently in the fourth to seventh decades (average age, 52 years).2,26–34 About 80–90% are stage I and only 5% are bilateral.2,26–34 Of note, metastatic mucinous tumors in the ovary often mimic primary ovarian mucinous neoplasms, particularly adenocarcinomas of the pancreas and large intestine (see Chapter 30).35 Microscopically, the metastatic tumor may appear deceptively ‘benign,’ ‘borderline,’ or malignant. It has been established that most, if not all, ovarian mucinous tumors associated with pseudomyxoma peritonei are secondary tumors, frequently low-grade appendiceal mucinous neoplasms (see later).36 Bilaterality is exceptional in stage I ovarian mucinous tumors; therefore, tumor involvement of both ovaries should raise the suspicion of metastatic carcinoma.
Macroscopic Features
On gross examination, the tumors average 19 cm in diameter, are usually cystic and multilocular, and contain mucinous fluid (Figure 26.12). Papillae and polypoid excrescences may line the cysts. MBTs of gastrointestinal type cannot be distinguished grossly from mucinous cystadenomas and cystadenocarcinomas. These tumors should be sampled extensively, particularly the solid portions, since variable degrees of epithelial proliferation and nuclear atypia (from benign to borderline, and to carcinoma) coexist frequently within an individual neoplasm.
Microscopic Features
Microscopically, MBTs of gastrointestinal type consist of cysts and glands lined by atypical epithelium of gastric pyloric type. The cysts may contain papillae that are typically thin and branching (Figure 26.13). The lining epithelium almost always contains goblet cells and may have argyrophil cells and occasional Paneth cells. The epithelial cells are usually stratified to two or three layers, nuclear atypia is mild to moderate, and mitotic figures vary from few to numerous. High-grade nuclear features are absent and stromal invasion is not seen (Figure 26.14). The overall appearance resembles that of a hyperplastic or adenomatous colonic polyp. Most tumors also contain foci of benign mucinous epithelium, which resembles endocervical or gastric epithelium. Occasionally, pools of extravasated mucin dissect into the stroma and may be associated with a histiocytic and foreign-body–giant-cell reaction (mucin granuloma) or may lack an inflammatory cell response (pseudomyxoma ovarii). Focal necrosis and acute inflammation are not uncommon.
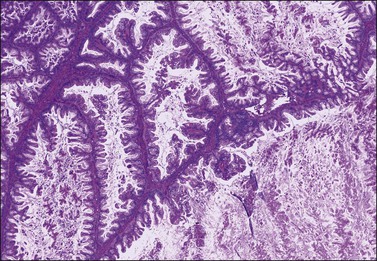
Figure 26.13 Mucinous cystic borderline tumors of intestinal type. Intraglandular proliferation of mucinous epithelium with filiform branching papillae. There is no stromal invasion.
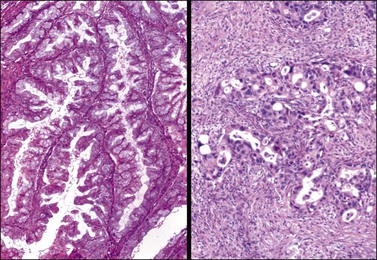
Figure 26.14 Mucinous borderline tumor (left) and mucinous carcinoma (right). The borderline tumor shows branching papillae with minimal stromal support, stratification into two or three cell layers, and mild nuclear atypia without stromal invasion. In contrast, the carcinoma exhibits severe nuclear atypia and obvious stromal invasion.
Noninvasive MBTs of gastrointestinal type may exhibit areas of epithelial cell proliferation of four or more layers, scattered foci of cribriform or stroma-free papillary architecture, and moderate (grade 2) or severe atypical (grade 3) nuclei (Figure 26.15). Whether tumors with such areas should be classified as noninvasive carcinomas or as borderline tumors has remained controversial for many years.13 Numerous studies,26–34 however, have shown that these tumors are almost always clinically benign, and we recommend classifying them as mucinous borderline tumors with intraepithelial carcinoma.2,37 Currently, the preferred and exclusive criterion for this diagnosis is marked nuclear atypia (grade 3); neither a cribriform pattern nor epithelial stratification of greater than three cell layers qualify.34,38 No minimum quantity of malignant-appearing epithelium is required for inclusion into this histologic category. The upper limit, however, merges imperceptibly with invasive carcinoma with an expansile growth pattern (see later) and has been arbitrarily established as 10 mm2. Because of their in situ malignant change, MBT with IEC requires more extensive sampling than pure intestinal-type MBTs to exclude stromal invasion. Cell pseudostratification and cribriform-like pattern caused by tangential sectioning characteristically lacks high-grade nuclear atypia and should not be interpreted as IEC.13
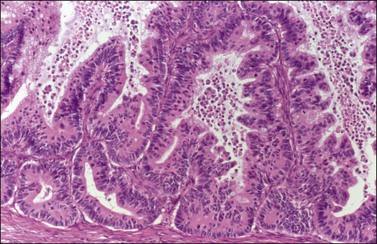
Figure 26.15 Mucinous borderline tumor with IEC. There is cell proliferation with glandular architectural complexity. The glands are lined by high-grade malignant nuclei with mitotic figures.
Ten per cent of intestinal-type MBTs contain one or more foci of stromal microinvasion (also arbitrarily defined as not exceeding 10 mm2). Individual microinvasive foci usually are <1 or 2 mm in greatest dimension. Their histologic appearances vary from small nests of tumor cells admixed with extracellular mucin in a normal ovarian stroma to irregular glands associated with a fibroblastic or edematous stroma, or tiny nests or isolated tumor cells within clear spaces. On the basis of less than 100 reported cases,20,21,30–34 the presence of these foci does not alter the favorable prognosis of intestinal-type MBT. Nevertheless, MBT with stromal microinvasion should be distinguished from microinvasive carcinoma. Whereas the microinvasive component and the adjacent glands of the former tumors exhibit only low-grade (borderline) nuclear atypia (Figure 26.16), the cells of microinvasive carcinomas usually contain grade 3 nuclei (Figure 26.17).13,32,38 Recently, a case of MBT with intraepithelial and microinvasive carcinoma associated with aggressive behavior has been reported.39 Stromal microinvasion may be difficult to distinguish from extruded neoplastic epithelium from an adjacent ruptured gland, particularly if accompanied by a mucin granuloma (Figure 26.4). Cytokeratin immunostaining reveals that mucin granulomas contain tumor cells more often than suspected on H&E.33
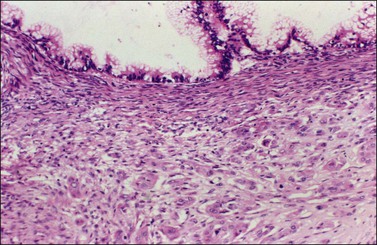
Figure 26.16 Mucinous borderline tumor with microinvasion. Stromal microinvasion by single epithelial cells and small clusters of such cells with abundant eosinophilic cytoplasm and minimal nuclear atypia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree