and Natalia Buza1
(1)
Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
Keywords
Malignant germ cell tumorsDysgerminomaYolk sac tumorChoriocarcinoma (non-gestational)Immature teratomaMature teratomaStruma ovariiCarcinoid tumorSomatic malignancy arising in mature teratomaIntroduction
Germ cell tumors account for approximately 30 % of primary ovarian neoplasms, comprising the second most common tumor type after epithelial tumors. However, vast majority of them are benign and the group represents only 2–3 % of ovarian malignancies. While the intraoperative diagnosis of the most common mature cystic teratoma (dermoid cyst) is usually straightforward, frozen section evaluation of malignant germ cell tumors is often challenging given their rarity and the clinical implications of the diagnosis. Most malignant germ cell tumors occur in young patients—children and young adults less than 30 years of age, hence preservation of fertility is of great importance. Thanks to recent advances in adjuvant chemotherapy, fertility-sparing surgery—intact removal of tumor, pelvic washings, surgical examination, and removal/biopsy of any suspicious areas from omentum and regional lymph nodes—now became the standard of care for this group of tumors [1–4]. The frozen section pathologist has a crucial role in recognizing these neoplasms and distinguishing them from their mimics—most importantly the more aggressive epithelial ovarian cancers—to guide appropriate surgical management.
The importance of the clinical presentation and laboratory findings as an aid in the frozen section diagnosis cannot be overemphasized in ovarian pathology in general, but this is especially true for germ cell tumors. The patient’s age, hormonal manifestations (e.g., precocious puberty), and serum tumor markers can provide important diagnostic clues and significantly narrow down the differential diagnosis during intraoperative consultation.
Dysgerminoma
Clinical features
Most common malignant germ cell tumor (approximately 50 % of all malignant germ cell tumors).
Occurs most often during the 2nd and 3rd decades with a mean patient age of 22 years.
Rapidly growing tumor; frequently presents with abdominal mass and pain.
May be an incidental finding, e.g., during pregnancy.
Serum LDH is often elevated.
Rarely may be associated with paraneoplastic hypercalcemia [5].
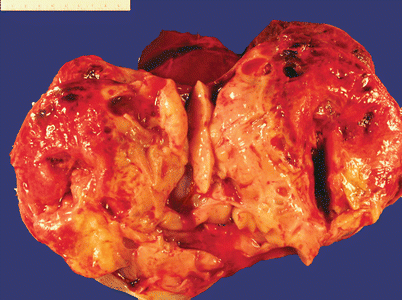
Gross pathology (Fig. 9.1)
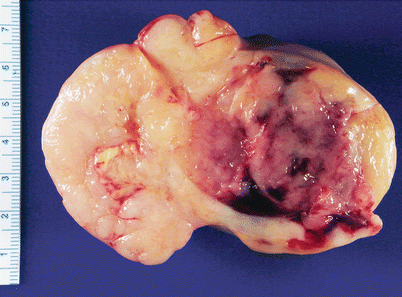
Fig. 9.1
Dysgerminoma. Note the solid, tan-yellow, fleshy cut surface with foci of hemorrhage and necrosis
Bilateral in 10–20 % of cases
Large, usually more than 10 cm in diameter [8]
Cut surface is solid, fleshy, and tan-yellow
Small foci of necrosis, hemorrhage, and cystic degeneration may be seen.
Microscopic features (Fig. 9.2)
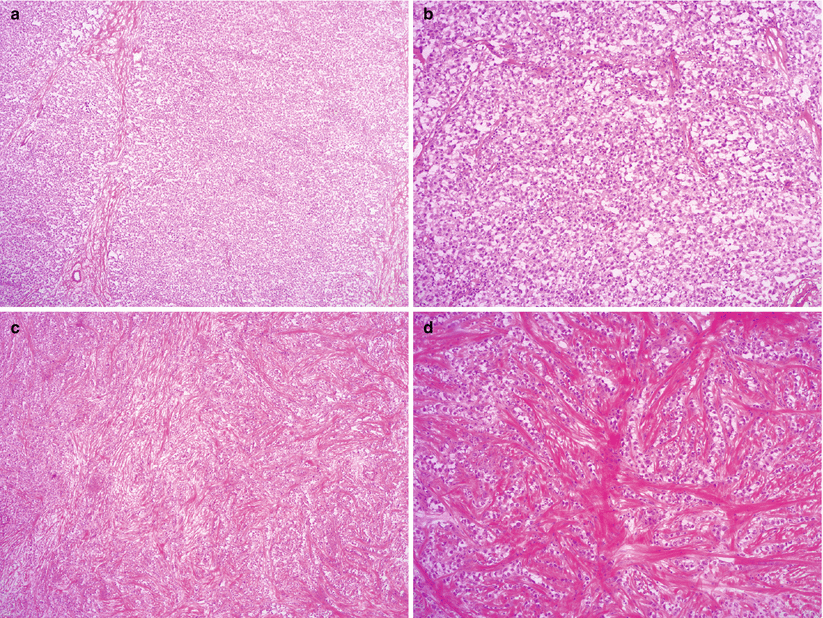
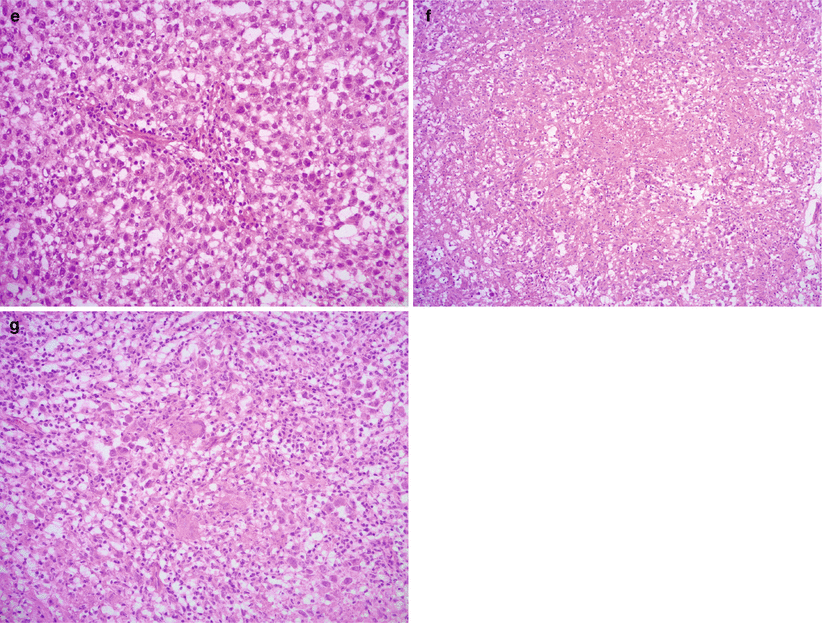
Fig. 9.2
Dysgerminoma, microscopic features. The tumor cells form solid sheets or nests, divided by fibrous septa with lymphocytic infiltrate (a, b). Marked stromal fibrosis and hyalinization may be seen (c, d). The tumor cells are relatively uniform, polygonal with prominent nucleoli and abundant eosinophilic or clear cytoplasm (e). Granulomatous reaction with multinucleated giant cells is present in some cases (f, g)
Solid nests, sheets, or cords of relatively uniform polygonal cells.
Abundant clear or eosinophilic cytoplasm with well-defined cell membranes.
Prominent nucleoli.
High mitotic activity.
Intersecting fibrous septae, infiltrated by small lymphocytes.
Ratio between tumor cells and fibrous stroma may vary, in some tumors hyalinized stroma may predominate
Sarcoid-like granulomas with multinucleated giant cells may be seen in up to 20 % of cases.
Syncytiotrophoblastic giant cells may rarely be seen.
May be associated with elevated serum beta human chorionic gonadotropin (hCG)
Focal necrosis and hemorrhage are not uncommon.
Calcifications may be present.
Differential diagnosis
Diffuse large B-cell lymphoma
Clear cell carcinoma
Yolk sac tumor
Embryonal carcinoma
Gonadoblastoma
Diagnostic pitfalls/key intraoperative consultation issues
Contralateral ovarian biopsy should be performed to rule out bilateral involvement by dysgerminoma or underlying gonadal dysgenesis and/or gonadoblastoma.
Bilateral oophorectomy is indicated for gonadal dysgenesis and gonadoblastoma.
Abundant calcifications should raise suspicion for gonadoblastoma.
Fertility-preserving surgery—unilateral salpingo-oophorectomy with peritoneal biopsies and lymph node sampling—is the standard first-line treatment [1, 9].
Other malignant germ cell tumors in the differential diagnosis—yolk sac tumor and embryonal carcinoma—have essentially the same surgical management.
Entities in the differential with the most significant impact on intraoperative management:
Diffuse large B-cell lymphoma
Primarily nonsurgical treatment.
Fresh tissue sample should be submitted for hematopathology—flow cytometry and molecular studies—workup.
Lymphoma cells are more polymorphous and have less cytoplasm than dysgerminoma.
Epithelial malignancies (e.g., clear cell carcinoma and small cell carcinoma)
More aggressive tumors, typically requiring more extensive staging—tumor debulking—surgery
Yolk Sac Tumor (YST)
Clinical features
Accounts for approximately 20 % of malignant germ cell tumors
Occurs during the 2nd and 3rd decades with a mean patient age of 16–19 years
Rapidly growing pelvic mass
Abdominal pain, may be due to torsion
Elevated serum alfa-fetoprotein (AFP)
Microscopic features (Fig. 9.4)
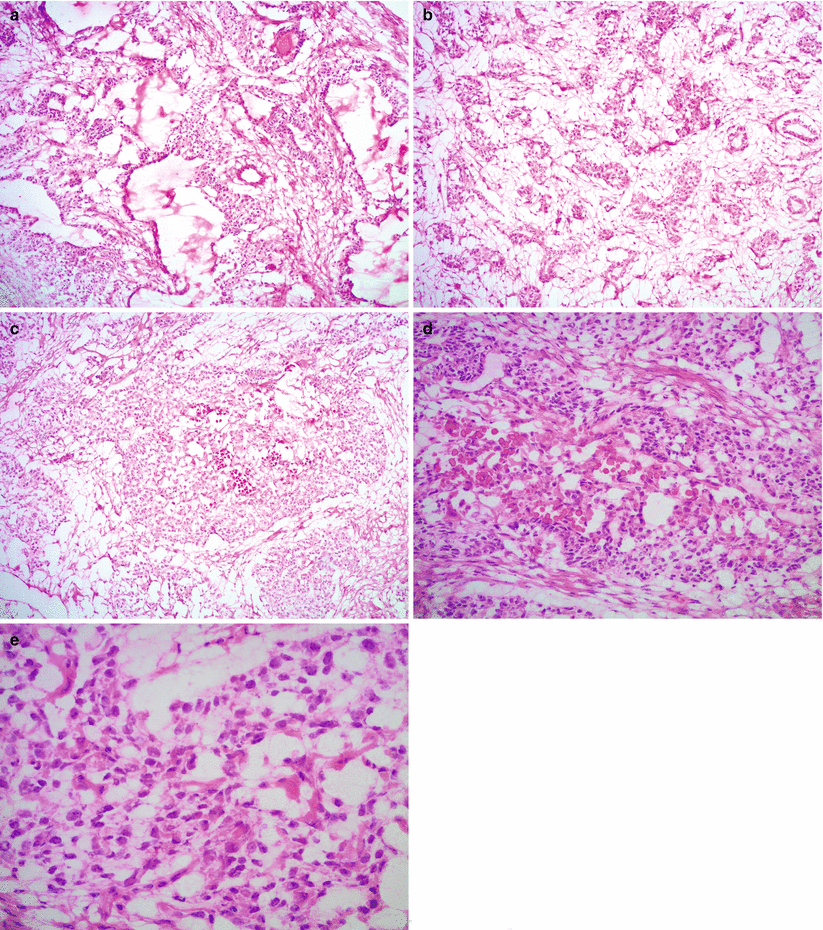
Fig. 9.4
Yolk sac tumor, microscopic features. The tumor shows various growth patterns, including reticular (a), alveolar-glandular (b), and solid architecture (c). The tumor cells have pleomorphic nuclei with prominent nucleoli and clear or pale eosinophilic cytoplasm containing hyaline globules (d, e)
Various different microscopic growth patterns, often within the same tumor:
Reticular pattern: most common, composed of microcysts and anastomosing network of irregular luminal structures
Endodermal sinus pattern with characteristic perivascular Schiller-Duval bodies, only seen in approximately 20 % of cases
Solid pattern
Alveolar-glandular pattern
Other rare patterns: polyvesicular vitelline, hepatoid, papillary, and myxomatous
Cytoplasm may be clear or pale eosinophilic.
Eosinophilic cytoplasmic or extracellular hyaline globules are often present.
Intestinal differentiation may be seen with goblet cells and mucin production.
Moderate to marked nuclear pleomorphism with prominent nucleoli and frequent mitotic figures.
Loose, myxoid stroma is often prominent in areas with alveolar-glandular pattern.
Necrosis and hemorrhage are common.
Differential diagnosis
Primary ovarian carcinomas
Endometrioid adenocarcinoma
Clear cell carcinoma
Other malignant germ cell tumors
Dysgerminoma
Embryonal carcinoma
Immature teratoma
Sertoli-Leydig cell tumor, especially retiform variant
Diagnostic pitfalls/key intraoperative consultation issues
For intraoperative surgical management, the most important entities in the differential are primary ovarian—endometrioid and clear cell—carcinomas:
Typically require more extensive staging—tumor debulking—surgery.
Endometrioid and clear cell ovarian carcinomas usually affect older patients, but there is some overlap in patient age with YST.
Both endometrioid and clear cell carcinomas are more uniform architecturally and may be associated with endometriosis.
Examination of multiple tissue blocks is useful in identifying the various different growth patterns, characteristic of YST.
Hyaline globules are nonspecific and may be also seen in other entities in the differential, e.g., clear cell carcinoma.
Distinction between YST and other malignant germ cell tumors on frozen section is not critical for intraoperative management.
Other malignant germ cell tumors in the differential have essentially the same intraoperative management: fertility-preserving surgery (unilateral salpingo-oophorectomy with peritoneal biopsies and lymph node sampling) [1].
Dysgerminomas are more uniform than YST, both at the architectural and cytological level.
Choriocarcinoma (Non-gestational)
Clinical features
Rare tumor, less than 1 % of malignant germ cell tumors
Symptoms may include:
Abdominal mass/pain, rarely hemoperitoneum
Isosexual pseudoprecocity
Vaginal bleeding
Elevated serum beta-hCG with positive pregnancy test
May mimic ectopic pregnancy clinically
Gross pathology
Typically unilateral
Large tumor with solid and cystic cut surface, often with hemorrhage and necrosis
Microscopic features
Bi- or triphasic appearance with sheets of mononuclear cytotrophoblast, intermediate-type trophoblast, and multinucleated syncytiotrophoblast.
Marked nuclear pleomorphism and frequent mitotic figures.
May be mixed with other malignant germ cell tumor components.
Necrosis and hemorrhage are often present.
Differential diagnosis
Gestational choriocarcinoma
Other malignant germ cell tumors (e.g., dysgerminoma, embryonal carcinoma, YST) with isolated syncytiotrophoblastic giant cells
Poorly differentiated ovarian carcinomas (somatic, non-germ cell origin) with trophoblastic differentiation
Diagnostic pitfalls/key intraoperative consultation issues
Distinction between choriocarcinoma and other malignant germ cell tumors on frozen section is not critical for intraoperative management.
Ovarian carcinomas of somatic origin with trophoblastic differentiation typically present in older patients, often postmenopausal, in contrast to choriocarcinoma.
Unlike choriocarcinoma, somatic (non-germ cell) ovarian carcinomas require aggressive surgery.
In postmenopausal patients—if the histological features are equivocal—additional tissue blocks may be sampled to help identifying the morphologically more typical somatic carcinoma component (e.g., poorly differentiated endometrioid adenocarcinoma), and a frozen section diagnosis of “carcinoma with probable trophoblastic differentiation” can be communicated.
Non-gestational (germ cell origin) and gestational choriocarcinomas are morphologically identical.
Gestational origin can be ruled out in preadolescent patients.
Distinction between the two pathogenetic entities is typically not crucial for intraoperative management.
Embryonal Carcinoma
Clinical features
Very rare tumor
Occurs in young patients (<30 years of age), with a mean age of 15 years [14]
Clinical presentation may include:
Abdominal mass/pain
Isosexual pseudoprecocity
Vaginal bleeding
Elevated serum AFP and beta-hCG (positive pregnancy test)
Gross pathology
Unilateral.
Mean tumor size is 15 cm.
Cut surface is solid tan-grey, often with foci of hemorrhage and necrosis.
Microscopic features
Sheets of large, polygonal cells.
Pleomorphic, hyperchromatic nuclei with prominent nucleoli.
High mitotic activity and numerous apoptotic bodies.
Abundant, amphophilic cytoplasm.
Syncytiotrophoblastic giant cells are often present.
Necrosis and hemorrhage are common.
May be mixed with other malignant germ cell tumor components.
Differential diagnosis
Other malignant germ cell tumors (e.g., dysgerminoma, choriocarcinoma, and YST)
Poorly differentiated ovarian carcinomas (somatic, non-germ cell origin)
Diagnostic pitfalls/key intraoperative consultation issues
Distinction between embryonal carcinoma and other malignant germ cell tumors on frozen section is not critical for intraoperative management.
The tumor cells in dysgerminoma are more uniform, and characteristic fibrovascular septae with lymphocytic infiltrate are present.
YST typically shows various growth patterns, compared to the relatively homogeneous morphology of embryonal carcinoma.
Poorly differentiated ovarian carcinomas of somatic (non-germ cell) origin typically present in older patients—often postmenopausal—in contrast to embryonal carcinoma and require extensive staging surgery.
Mixed Germ Cell Tumor
Occurs in children and young adults.
Admixture of two or more types of malignant germ cell tumors.
Most common combination is dysgerminoma and yolk sac tumor, but any combination of tumor types may occur.
Precise identification of different histological components of a mixed germ cell tumor is most important for adjuvant therapy and prognostication; however, it is not critical for intraoperative management, and a frozen section diagnosis of “malignant germ cell tumor, favor/probable mixed components” is usually sufficient.
Teratomas
Mature Teratoma/Mature Cystic Teratoma/Dermoid Cyst
Clinical features
Very common, accounting for up to 44 % of all ovarian tumors [15, 16] and over 95 % of ovarian teratomas
May occur at any age, but most commonly during the reproductive years
May be asymptomatic or presents with pelvic mass/pain
May undergo torsion
Often an incidental finding—on imaging studies or during unrelated surgery
Rarely may be associated with autoimmune hemolytic anemia or encephalitis (anti-N-methyl-D-aspartate (NMDA) receptor encephalitis)
Gross pathology (Fig. 9.5)
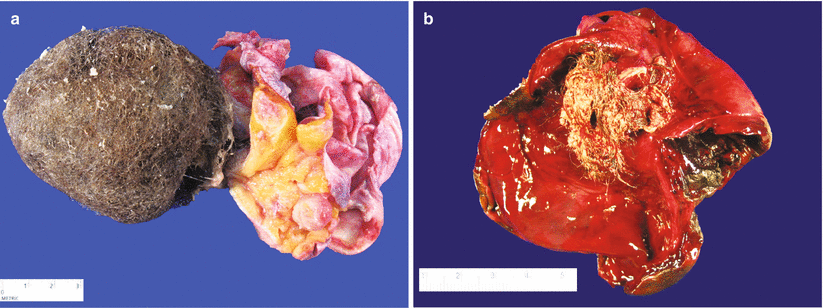
Fig. 9.5
Mature cystic teratoma (dermoid cyst). The cut surface typically shows a unilocular cyst filled with hair and sebaceous material. Solid areas may also be present (a, right side of image). Hemorrhagic infarction may be seen as a result of torsion (b)
Bilateral in 10–15 % of cases.
Almost always cystic—usually unilocular cyst filled with sebaceous material (typically still liquid at the time of frozen section) and hair.
Solid nodule (Rokitansky protuberance) is also commonly present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree