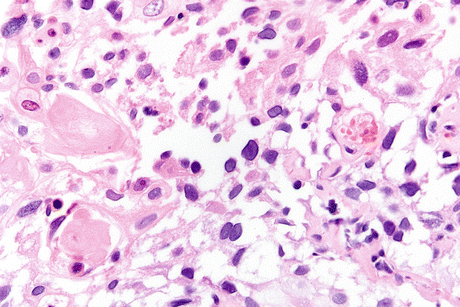
Fig. 8.1
Schistosoma hematobium eggs in cytologic preparations. (a) S. hematobium egg shows an oval structure with a terminal spine (at the end of an oval egg) surrounded by urothelial and inflammatory cells (Voided urine, TP, high mag.) (b) Well-displayed S. hematobium egg in an older filter preparation, note the lancet-shaped terminal and internal egg detail. Preserved squamous cells are present in the background. In the inset a S. hematobium egg is surrounded by inflammatory cells and debris (Voided urine; Main Image: Filter Preparation, high mag; Inset: CS, medium mag.)
Definition
SqCC of the urinary tract is a malignant neoplasm that shows exclusively squamous differentiation, without associated urothelial or glandular elements.
Diagnostic Criteria
Cellular specimen with numerous individual and nests of squamous cells (Figs. 8.2 and 8.3).
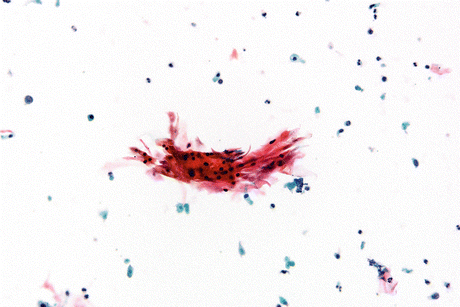
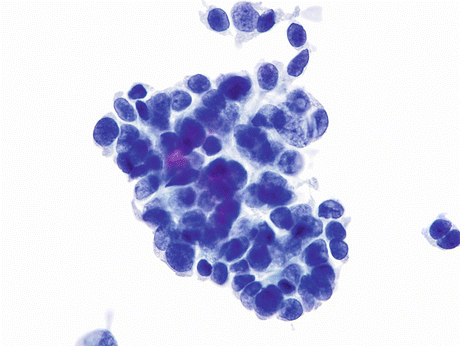
Fig. 8.2
Squamous cell carcinoma (SqCC) of the urinary bladder showing a nest of elongated keratinized “fiber” cells in a background of inflammatory cells and some keratotic debris (Voided urine, TP, low mag.)
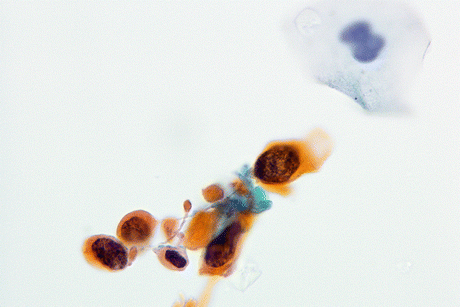
Fig. 8.3
SqCC of urinary bladder with coarse dysplastic nuclear features. Compare the size and staining of the neoplastic cell nuclei to the umbrella cell in the upper right hand corner of the image (Voided urine, TP, medium mag.)
Tumor cells are large, polygonal with keratinized cytoplasm, sharp borders and mildly to markedly atypical hyperchromatic nuclei (Figs. 8.3 and 8.4). Fiber and tadpole cells, squamous pearls and “cell in cell” arrangements may be present.
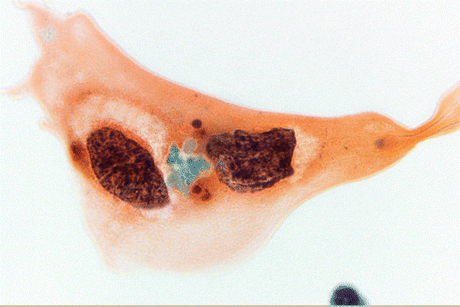
Fig. 8.4
Keratinizing SqCC cells of the urinary bladder display large, polygonal tumor cells with keratinized orangeophilic cytoplasm, sharp borders, and mildly to markedly atypical hyperchromatic nuclei (Voided urine, TP, high mag.)
Background may show plaques and fragments (“ghost cells”) of anucleated squamous cells, small atypical parakeratotic cells, necrosis, red blood cells, and neutrophils (Figs. 8.2 and 8.5).

Fig. 8.5
Keratinizing SqCC showing plaque/fragment of anucleated squamous cells (“ghost cells”). Note one nucleated atypical squamous cell in contrast to the degenerating nuclei in the other cells (Voided urine, TP, medium mag.)
Non-keratinizing malignant cell groups with metaplastic appearance may be present (Fig. 8.6).
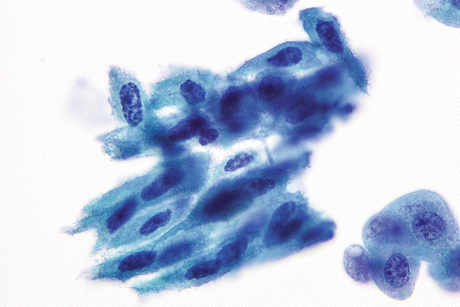
Fig. 8.6
Non-keratinizing SqCC of urinary bladder shows metaplastic type of cells with rigid basophilic cytoplasm and hyperchromatic angulated malignant nuclei (Voided urine, TP, medium mag.)
Liquid-Based Preparations (LBP)
Explanatory Notes
Explanatory Note 1: Cytologic diagnosis of well-to-moderately differentiated SqCC of the bladder is usually straightforward. Diagnostic difficulties may be encountered for well-differentiated SqCC, which may be difficult to diagnose as malignant, and for poorly differentiated SqCC, which may be difficult to diagnose as squamous.
Explanatory Note 2: In well-differentiated tumors, a careful search may disclose the presence of squamous cells showing nuclear enlargement and hyperchromasia or prominent nuclear membrane irregularity as well as necrotic debris [9]. Occasionally, these tumors are diagnosed as “atypical squamous cells”. The differential diagnosis of the latter includes a variety of benign, dysplastic or malignant lesions, including, squamous metaplasia, squamous papilloma, condyloma acuminatum, dysplasia, in situ and invasive SqCC of the bladder as well as contamination from such lesions in the lower female genital tract and UC with divergent squamous differentiation. Since the underlying rate of malignancy associated with “atypical squamous cells” in urine cytology is about 20 % [9], cystoscopy and biopsy may be indicated for these patients. Colposcopy may be required to exclude lower female genital tract origin.
Explanatory Note 3: The diagnosis of SqCC should be accompanied by a disclaimer stating that UC with divergent squamous differentiation cannot be excluded. The diagnosis of pure SqCC should be based on resection specimens. S100P, GATA3, and uroplakin III [10] are, in the order of decreasing sensitivity, immunohistochemical markers that can be used to demonstrate urothelial differentiation.
Adenocarcinoma
Background
Adenocarcinoma (AdCa) is the third most common malignant neoplasm of the urinary tract, accounting for 0.5–2.5 % of all primary bladder malignancies and includes vesical AdCa and urachal AdCa. The latter develops within urachal remnants located in the dome of the bladder and often secondarily involves the bladder [7, 11]. Secondary involvement of the bladder and upper urinary tract by direct extension or metastasis from AdCa from other organs is unusual, but is more common than primary urinary tract AdCa. Risk factors for primary AdCa include bladder exstrophy and cystitis glandularis, intestinal type [7, 12].
Primary bladder and urachal AdCas have similar morphology and can be classified as mucinous, enteric, signet ring cell, mixtures of the above types, and AdCa with no distinctive features, i.e., not otherwise specified (AdCa, NOS). Urachal AdCas are more frequently mucinous (“colloid”), whereas non-urachal AdCa are more frequently AdCa, NOS (Figs. 8.8), and signet ring cell AdCa, which may explain their worse prognosis [13]. While primary bladder AdCas are similar to UCs in their age and gender distribution, urachal AdCas occur in a much younger patient population with a median age of around 50 years and have no gender predilection. Clinical symptoms at presentation are hematuria, dysuria, urinary frequency, and rarely mucosuria.
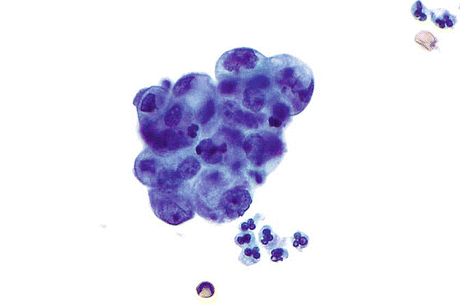

Fig. 8.8
Adenocarcinoma, not otherwise specified (AdCa, NOS) displays a cluster of cells with eccentrically placed irregular nuclei, prominent nucleoli, and finely vacuolated cytoplasm. In an individual fragment it is difficult to determine if the apparent vacuolization is due to glandular secretion or degeneration, but the observation of many such fragments results in a conclusion of glandular differentiation (Voided urine, TP, high mag.)
Definition
Primary AdCa of the urinary bladder is a malignant neoplasm derived from metaplastic urothelium showing histologically pure glandular differentiation. Urachal AdCa develops within urachal remnants.
Criteria
Cellularity of cytologic samples is variable.
Enteric (colonic-type) AdCa, (Fig. 8.9) show columnar cell clusters and single degenerated cells in a background of necrosis and mucin. Nuclei are large, vesicular or hyperchromatic, with irregular shapes and visible or prominent nucleoli. The cytoplasm may be vacuolated (Fig. 8.10).
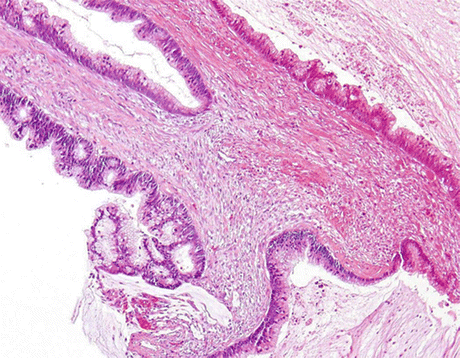
Fig. 8.9
Resected bladder with enteric (colonic) AdCa, exhibiting neoplastic glandular epithelium with tall columnar cells with elongated pencil-shaped nuclei and background mucin (H&E, low mag.)
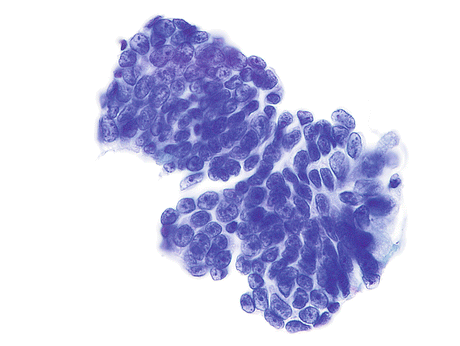
Fig. 8.10
Enteric (colonic-type) AdCa of the urinary bladder demonstrates a cluster of cells. Nuclei are large, columnar to round, irregular, hyperchromatic with thick nuclear membranes and prominent nucleoli. The cytoplasm is scant and vacuolated (Voided urine, TP, high mag.)
Mucinous (colloid) AdCa show rounded three-dimensional clusters of crowded, rather bland cells with small to moderate amounts of lacy cytoplasm with occasional mucin vacuoles and medium-sized nuclei with visible nucleoli. Mucin is present in the background.
Signet ring cell carcinoma displays cells with a large cytoplasmic mucin-containing vacuole that may appear optically clear or finely vacuolated and pushes the crescent-shaped hyperchromatic nucleus to the periphery of the cell (Fig. 8.11).
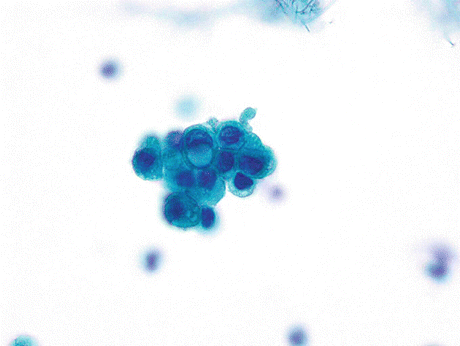
Fig. 8.11
Signet ring cell carcinoma of the urinary bladder displays a cluster of cells with one cell showing a crescent-shaped hyperchromatic nucleus pushed to the periphery of the cell by a large cytoplasmic mucin-containing vacuole (Voided urine, SP, high mag.)
Clear cell AdCa has cells with abundant vacuolated cytoplasm and centrally located nuclei that may be present in clusters with hobnail configuration (Fig. 8.12).
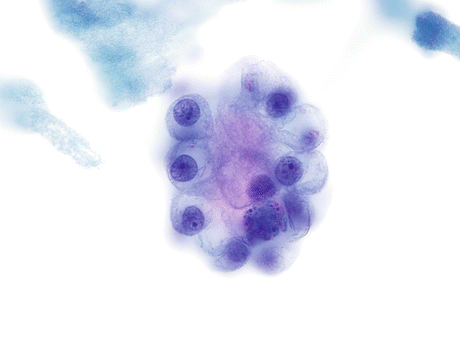
Fig. 8.12
Clear cell AdCa of the urinary bladder is characterized by a cluster of cells with projecting cytoplasm in a “hobnail configuration”. The abundant vacuolated cytoplasm and centrally located nuclei with prominent nucleoli complete the features of this subtype of cancer (Voided urine, TP, high mag.)
Explanatory Notes
Explanatory Note 1: The cytomorphology of AdCa in urinary tract cytology specimens differs according to the type of AdCa, as seen above and usually can be readily diagnosed as malignant [11–13].
Differential diagnosis of well-differentiated primary AdCa of the bladder includes glandular cells from a fistula with the vagina or the gastrointestinal tract, cystitis glandularis, intestinal metaplasia, nephrogenic metaplasia/adenoma, and villous adenoma [7, 14].
Explanatory Note 2: The exclusion of UCs with glandular differentiation may be difficult, and may require the use of immunostains to demonstrate a malignant urothelial component that may be positive (with decreasing frequency) for S100P, p63, and GATA3 [15].
However, the most difficult task is to rule out secondary involvement of the bladder by AdCa from other organs, either by direct extension (colorectal, prostatic, and gynecologic) or by hematogenous metastasis (breast, pancreatic, gastric, and pulmonary). Clinical and imaging correlation and immunostains are most effective in this regard.
Explanatory Note 3: Since most primary AdCas of the bladder have an enteric (colonic) or signet ring cell phenotype, it is important to rule out secondary involvement from a colorectal primary. Although urinary tract AdCas typically express markers of intestinal differentiation (villin, CK20, CEA, and CDX2) and may even harbor KRAS mutations, they lack nuclear beta-catenin expression, which is present in over 80 % of colorectal AdCa, and are frequently positive for CK7 [11]. GATA3 expression may be seen in signet ring cell carcinomas of the bladder, but not in metastatic gastric signet ring cell carcinomas [15]. Clear cell AdCa have to be differentiated from renal cell carcinomas (RCCs) by using renal cell markers, PAX-8, CD10, and CA IX. Prostatic ductal AdCa frequently extends into the bladder or urethra and can show cytological features similar to those of primary urinary tract AdCa from which they can be differentiated due to their PSA immunopositivity [11, 12, 14].
Neuroendocrine Tumors
Two types of neuroendocrine (NE) tumors, with diverse clinicopathological features and outcome, occur in the urinary bladder including carcinoid tumor and neuroendocrine carcinoma (NEC). Both of these can involve the kidney, prostate, and urinary bladder and are morphologically, histochemically, immunohistochemically, and ultrastructurally similar to their counterpart in other organs such as lungs and GI tract. NEC of the urinary bladder with large cell (LCNEC) and small cell NEC morphology are rare entities, accounting for less than 1 % of urinary bladder malignancies. The latter is more common.
The histogenesis of NEC of the bladder remains to be elucidated. Approximately half the reported cases have a mixture with other histologic subtypes in which UC predominates. This supports the most widely accepted view that the NEC of the bladder originates in a pluripotential stem cell that can differentiate into various cell types suggesting a common clonal origin of the NE, urothelial, squamous, or adenocarcinoma component.
Carcinoid. Primary carcinoid tumors of the urinary bladder are exceedingly rare with fewer than 20 pure histologically documented cases reported in the literature. Rarely, carcinoids may be associated with UC. The average age is around 55 years and present with hematuria. The tumor occurs in the trigone and bladder neck as a polypoid or smooth small submucosal nodule and is mostly cured by simple resection. Cytology is similar to carcinoid tumors elsewhere. The prognosis of carcinoid is very favorable; however, it should be distinguished from paraganglioma/pheochromocytoma, NECs, lymphoma, and UC [16].
Small Cell Carcinoma and Large Cell Neuroendocrine Carcinoma
Background
Extrapulmonary small cell carcinoma (SmCC) is rare and can occur at diverse sites including the bladder and accounts for less than 1 % of all urinary bladder carcinomas. Like pulmonary SmCC, it is closely associated with tobacco smoking and is also morphologically and prognostically similar (Fig. 8.13). The most favored histogenesis of the tumor is origin from basally located multipotential stem cells that can differentiate into various cell types. This origin may explain the frequent (more than 50 % of cases) association of SmCC with other carcinomatous components, such as UC, SqCC, AdCa, sarcomatoid carcinoma, or mixtures of these components. Tumors with pure or mixed histologic patterns are classified as SmCC rather than UC, SqCC, or AdCa with NE differentiation [17]. Clinical and other demographic features as well as gross appearance of SmCC are similar to those seen in patients with conventional UC of the bladder.
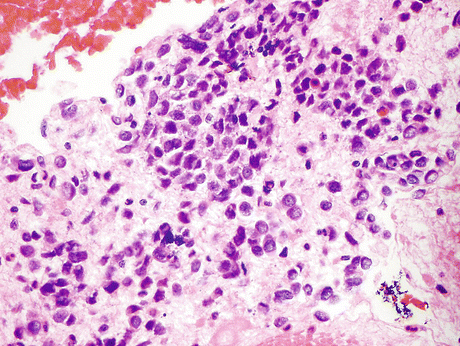

Fig. 8.13
Resected primary small cell carcinoma (SmCC) of the urinary bladder confirms the cytology described in Fig. 8.16. These tumors are characterized by small highly irregular nuclei 2–3 times the size of a lymphocyte nucleus. Tight nuclear groups frequently exhibit nuclei pushing into one another (“nuclear molding”). Histomorphology is similar to SmCC from other sites (H&E, high mag.)
Definition of SmCC
Malignant tumor of NE origin is similar to SmCC of lung and other sites. Tumor cells are positive for NE markers (synaptophysin, chromogranin, CD56) (Fig. 8.14).
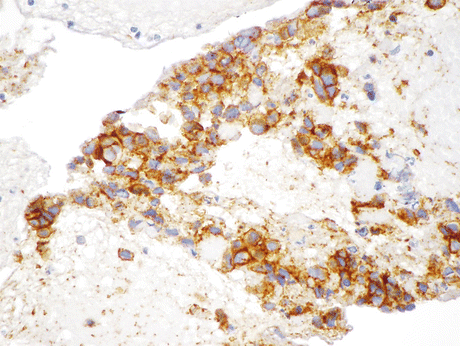

Fig. 8.14
Positive immunostain for CD56 (neural cell adhesion molecule, also found associated with skeletal muscle and NK cells) performed on the resected SmCC from the case described in Fig. 8.13 indicates the tumor’s NE lineage (Biopsy, IHC, high mag.)
Criteria
Cellularity is usually moderate to high.
Background is hemorrhagic and necrotic with single-cell necrosis (apoptosis), isolated or small groups of small, undifferentiated malignant cells, mitoses, and numerous polymorphonuclear leukocytes.
Cells are arranged singly, in linear pattern, rosettes, loosely or tightly cohesive clusters (Fig. 8.15).
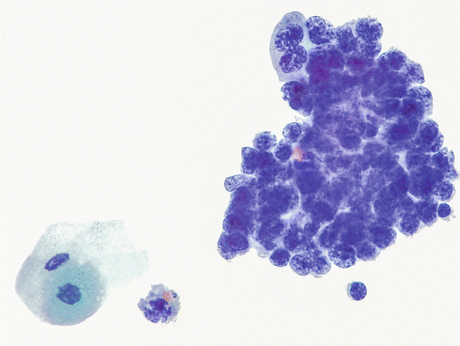
Fig. 8.15
A case of SmCC (histology depicted in Figs. 8.13 and 8.14) displays cells arranged in a tightly cohesive cluster. The background is clean and the typical crush artifact in these delicate cells is not evident. Tumor cells show a high nucleus-cytoplasmic ratio, moderately enlarged (compare to a lymphocyte at 5 o’clock position) round to oval irregular nuclei with hyperchromatic to smudged chromatin and absent or inconspicuous nucleoli. Nuclear overlap is more pronounced than molding. Cytoplasm is scant. Note benign umbrella cell in the lower left corner (Voided urine, TP, high mag.)
Tumor cells are round to oval or irregular and small to medium in size (2–3 times the size of lymphocytes).
Nuclei are small to oval, hyperchromatic with finely granular evenly distributed or smudged chromatin, ill-defined membranes, prominent molding, and display crush artifact. Nucleoli are absent or inconspicuous (Fig. 8.16).