Opioid peptides released from nerve endings modulate transmission in the brain and spinal cord and in primary afferents via their interaction with specific receptors. Many of the pharmacologic actions of opiates and synthetic opioid drugs are effected via their interactions with endogenous opioid peptide receptors.
High-Yield Terms to Learn
Opiate A drug derived from alkaloids of the opium poppy Opioid The class of drugs that includes opiates, opiopeptins, and all synthetic and semisynthetic drugs that mimic the actions of the opiates Opioid peptides Endogenous peptides that act on opioid receptors Opioid agonist A drug that activates some or all opioid receptor subtypes and does not block any Partial agonist A drug that can activate an opioid receptor to effect a submaximal response Opioid antagonist A drug that blocks some or all opioid receptor subtypes Mixed agonist-antagonist A drug that activates some opioid receptor subtypes and blocks other opioid receptor subtypes
Classification
The opioid analgesics and related drugs are derived from several chemical subgroups and may be classified in several ways.
Spectrum of Clinical Uses
Opioid drugs can be subdivided on the basis of their major therapeutic uses (eg, analgesics, antitussives, and antidiarrheal drugs).
Strength of Analgesia
On the basis of their relative abilities to relieve pain, the analgesic opioids may be classified as strong, moderate, and weak agonists. Partial agonists are opioids that exert less analgesia than morphine, the prototype of a strong analgesic, or full agonist.
Ratio of Agonist to Antagonist Effects
Opioid drugs may be classified as agonists (receptor activators [full or partial]), antagonists (receptor blockers), or mixed agonist-antagonists, which are capable of activating one opioid receptor subtype and blocking another subtype.
Pharmacokinetics
Absorption and Distribution
Most drugs in this class are well absorbed when taken orally, but morphine, hydromorphone, and oxymorphone undergo extensive first-pass metabolism. In most cases, opioids can be given parenterally, and sustained-release forms of some drugs are now available, including morphine and oxycodone. Opioid drugs are widely distributed to body tissues. They cross the placental barrier and exert effects on the fetus that can result in both respiratory depression and, with continuous exposure, physical dependence in neonates.
Metabolism
With few exceptions, the opioids are metabolized by hepatic enzymes, usually to inactive glucuronide conjugates, before their elimination by the kidney. However, morphine-6-glucuronide has analgesic activity equivalent to that of morphine, and morphine-3-glucuronide (the primary metabolite) is neuroexcitatory. Codeine, oxycodone, and hydrocodone are metabolized by cytochrome CYP2D6, an isozyme exhibiting genotypic variability. In the case of codeine, this may be responsible for variability in analgesic response because the drug is demethylated by CYP2D6 to form the active metabolite, morphine. The ingestion of alcohol causes major increases in the peak serum levels of several opioids including hydromorphone and oxymorphone. Meperidine is metabolized to normeperidine, which may cause seizures at high plasma levels. Depending on the specific drug, the duration of their analgesic effects ranges from 1-2 h (eg, fentanyl) to 6-8 h (eg, buprenorphine). However, long-acting formulations of some drugs may provide analgesia for 24 h or more. The elimination half-life of opioids increase in patients with liver disease. Remifentanil, a congener of fentanyl, is metabolized by plasma and tissue esterases and has a very short half-life.
Mechanisms of Action
Receptors
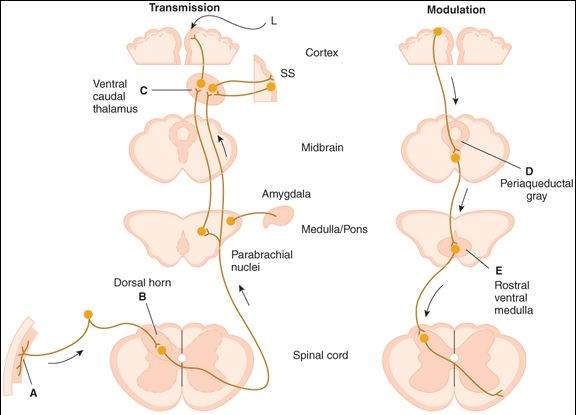
Many of the effects of opioid analgesics have been interpreted in terms of their interactions with specific receptors for endogenous peptides in the CNS and peripheral tissues. Certain opioid receptors are located on primary afferents and spinal cord pain transmission neurons (ascending pathways) and on neurons in the midbrain and medulla (descending pathways) that function in pain modulation (Figure 31-1). Other opioid receptors that may be involved in altering reactivity to pain are located on neurons in the basal ganglia, the hypothalamus, the limbic structures, and the cerebral cortex. Three major opioid receptor subtypes have been extensively characterized pharmacologically:  ,
, 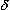 , and
, and  receptors. All 3 receptor subtypes appear to be involved in antinociceptive and analgesic mechanisms at both spinal and supraspinal levels. The
receptors. All 3 receptor subtypes appear to be involved in antinociceptive and analgesic mechanisms at both spinal and supraspinal levels. The  -receptor activation plays a major role in the respiratory depressant actions of opioids and together with
-receptor activation plays a major role in the respiratory depressant actions of opioids and together with  -receptor activation slows gastrointestinal transit;
-receptor activation slows gastrointestinal transit;  -receptor activation also appears to be involved in sedative actions;
-receptor activation also appears to be involved in sedative actions; 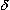 -receptor activation may play a role in the development of tolerance.
-receptor activation may play a role in the development of tolerance.
FIGURE 31-1
Putative sites of action of opioid analgesics (darker color). On the left, sites of action on the pain transmission pathway from the periphery to the higher centers are shown. (A) Direct action of opioids on inflamed or damaged peripheral tissues. (B) Inhibition also occurs in the spinal cord. (C) Possible sites of action in the thalamus. Different thalamic regions project to somatosensory (SS) or limbic (L) cortex. Parabrachial nuclei (medulla/pons) project to the amygdala. On the right, actions of opioids on pain-modulating neurons in the midbrain (D) , rostral ventral medulla (E) , and the locus coeruleus indirectly control pain transmission pathways by enhancing descending inhibition tothe dorsal horn.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 10th ed. McGraw-Hill, 2007.)
Opioid Peptides
Opioid receptors are thought to be activated by endogenous peptides under physiologic conditions. These peptides, which include endorphins such as 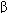 -endorphin, enkephalins, and dynorphins are synthesized in the soma and transported to the nerve endings where they accumulate in synaptic vesicles. On release from nerve endings, they bind to opioid receptors and can be displaced from binding by opioid antagonists. Endorphins have highest affinity for
-endorphin, enkephalins, and dynorphins are synthesized in the soma and transported to the nerve endings where they accumulate in synaptic vesicles. On release from nerve endings, they bind to opioid receptors and can be displaced from binding by opioid antagonists. Endorphins have highest affinity for  receptors, enkephalins for
receptors, enkephalins for 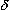 receptors, and dynorphins for
receptors, and dynorphins for  receptors. Although it remains unclear whether these peptides function as classic neurotransmitters, they appear to modulate transmission at many sites in the brain and spinal cord and in primary afferents. Opioid peptides are also found in the adrenal medulla and neural plexus of the gut.
receptors. Although it remains unclear whether these peptides function as classic neurotransmitters, they appear to modulate transmission at many sites in the brain and spinal cord and in primary afferents. Opioid peptides are also found in the adrenal medulla and neural plexus of the gut.
Ionic Mechanisms
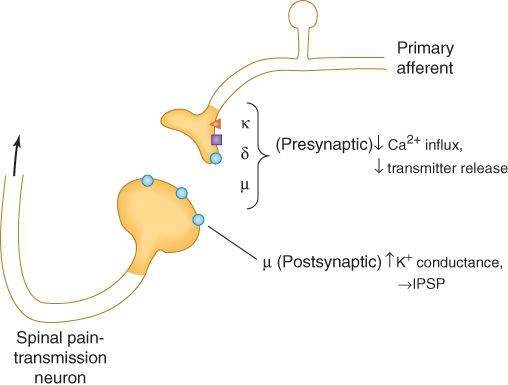
Opioid analgesics inhibit synaptic activity partly through direct activation of opioid receptors and partly through release of the endogenous opioid peptides, which are themselves inhibitory to neurons. All 3 major opioid receptors are coupled to their effectors by G proteins and activate phospholipase C or inhibit adenylyl cyclase. At the postsynaptic level, activation of these receptors can open potassium ion channels to cause membrane hyperpolarization (inhibitory postsynaptic potentials). At the presynaptic level, opioid receptor activation can close voltage-gated calcium ion channels to inhibit neurotransmitter release (Figure 31-2). Presynaptic actions result in the inhibition of release of multiple neurotransmitters, including acetylcholine (ACh), norepinephrine, serotonin, glutamate, and substance P.
FIGURE 31-2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree