II. Chemical Structure of Opium Alkaloids. The active components of opium can be divided into two distinct chemical classes: phenanthrenes and benzylisoquinolines. The principal phenanthrene alkaloids present in opium are morphine, codeine, and thebaine (Fig. 7-1).
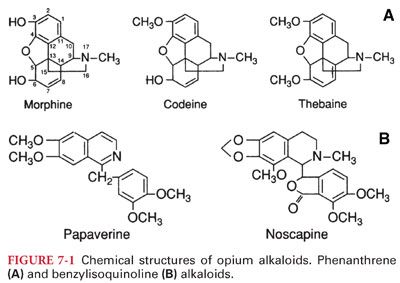
A. Semisynthetic Opioids. Simple modification of the morphine molecule yields many derivative compounds with differing properties. Substitution of a methyl group for the hydroxyl group on carbon 3 results in methylmorphine (codeine) and substitution of acetyl groups on carbons 3 and 6 results in diacetylmorphine (heroin).
B. Synthetic Opioids. Synthetic opioids contain the phenanthrene nucleus of morphine but are manufactured by synthesis rather than chemical modification of morphine.
1. Fentanyl, sufentanil, alfentanil, and remifentanil are synthetic opioids that are widely used to supplement general anesthesia or as primary anesthetic drugs in very high doses (Fig. 7-2).

2. The major pharmacodynamic differences between these drugs are potency and rate of equilibration between the plasma and the site of drug effect (biophase).
III. Mechanism of Action
A. Opioids act as agonists at specific opioid receptors at presynaptic and postsynaptic sites in the central nervous system (mainly the brainstem and spinal cord) as well as in the periphery.
B. These opioid receptors normally are activated by three endogenous peptide opioid receptor ligands known as enkephalins, endorphins, and dynorphins. (Opioids mimic the actions of these endogenous ligands by binding to opioid receptors, resulting in activation of pain-modulating [antinociceptive] systems.)
1. The principal effect of opioid receptor activation is a decrease in neurotransmission that occurs largely by presynaptic inhibition of neurotransmitter release (acetylcholine, dopamine, norepinephrine, substance P).
2. The intracellular biochemical events initiated by occupation of opioid receptors with an opioid agonist are characterized by increased potassium conductance (leading to hyperpolarization), calcium channel inactivation, or both, which produce an immediate decrease in neurotransmitter release.
IV. Opioid Receptors (Table 7-2)

A. μ Receptors are principally responsible for supraspinal and spinal analgesia.
B. Respiratory depression characteristic of μ receptor activation is less prominent with κ receptor activation, although dysphoria and diuresis may accompany activation of these receptors.
1. κ Receptor–mediated analgesia may be less effective for high-intensity painful stimulation than μ opioid–mediated.
2. Opioid agonist–antagonists often act principally on κ receptors.
V. Common Opioid Side Effects. All opioids possess similar side effects that vary only in degree.
A. Cardiovascular System
1. Morphine, even in large doses, given to supine and normovolemic patients is unlikely to cause direct myocardial depression or hypotension.
a. The same patients changing from a supine to a standing position, however, may manifest orthostatic hypotension and syncope, presumably reflecting morphine-induced impairment of compensatory sympathetic nervous system responses.
b. Morphine can also evoke decreases in systemic blood pressure due to drug-induced bradycardia (stimulation of the vagal nuclei in the medulla, direct depressant effect on the sinoatrial node) or histamine release (not all patients respond to morphine infusion with the release of histamine, emphasizing the individual variability). In contrast to morphine, the infusion of fentanyl does not cause release of histamine in any patient.
2. Morphine does not sensitize the heart to catecholamines or otherwise predispose to cardiac dysrhythmias as long as hypercarbia or arterial hypoxemia does not result from ventilatory depression.
3. During anesthesia, opioids are commonly administered with inhaled or intravenous anesthetics to ensure amnesia.
a. The combination of an opioid agonist such as morphine or fentanyl with nitrous oxide results in cardiovascular depression (decreased cardiac output and systemic blood pressure plus increased cardiac filling pressures), which does not occur when either drug is administered alone.
b. Decreases in systemic vascular resistance and systemic blood pressure may accompany the combination of an opioid and a benzodiazepine, whereas these effects do not accompany the administration of either drug alone.
4. Opioids have been increasingly recognized as playing a role in protecting the myocardium from ischemia.
B. Ventilation
1. All opioid agonists produce dose-dependent and gender-specific depression of ventilation, primarily through an agonist effect at mu2 receptors, leading to a direct depressant effect on brainstem ventilation centers. Because analgesic and ventilatory effects of opioids occur by similar mechanisms, it is assumed that equianalgesic doses of all opioids will produce some degree of ventilatory depression and reversal of ventilatory depression with an opioid antagonist always involves some reversal of analgesia.
a. Opioid-induced depression of ventilation is characterized by decreased responsiveness of these ventilation centers to carbon dioxide as reflected by an increase in the resting Paco2 and displacement of the carbon dioxide response curve to the right.
b. Death from an opioid overdose is almost invariably due to depression of ventilation.
2. Clinically, depression of ventilation produced by opioids manifests as a decreased frequency of breathing that is often accompanied by a compensatory increase in tidal volume (incompleteness of this compensatory increase in tidal volume is evidenced by predictable increases in the Paco2).
C. Central Nervous System
1. In the absence of hypoventilation, opioids decrease cerebral blood flow and possibly intracranial pressure (ICP) (use with caution in patients with head injury).
2. Rigidity. Rapid intravenous (IV) administration of large doses of an opioid (particularly fentanyl and its derivatives as used in cardiac surgery) can lead to generalized skeletal muscle rigidity.
3. Sedation. Postoperative titration of morphine frequently induces sedation that precedes the onset of analgesia. The usual recommendation for morphine titration includes a short interval between boluses (5 to 7 minutes) to allow evaluation of its clinical effect.
4. Nausea and Vomiting. Opioid-induced nausea and vomiting are caused by direct stimulation of the chemoreceptor trigger zone in the floor of the fourth ventricle. Morphine may also cause nausea and vomiting by increasing gastrointestinal secretions and delaying passage of intestinal contents toward the colon.
5. Placental Transfer. Opioids are readily transported across the placenta (depression of the neonate can occur).
6. Overdose
a. The principal manifestation of opioid overdose is depression of ventilation, manifesting as a slow breathing frequency, which may progress to apnea (triad of miosis, hypoventilation, and coma should suggest overdose with an opioid).
b. Treatment of opioid overdose is mechanical ventilation of the patient’s lungs with oxygen and administration of an opioid antagonist such as naloxone (may precipitate acute withdrawal in dependent patients).
7. Pharmacodynamic tolerance and physical dependence with repeated opioid administration are characteristics of all opioid agonists and are among the major limitations of their clinical use.
a. Physical dependence on morphine usually requires about 25 days to develop.
b. When physical dependence is established, discontinuation of the opioid agonist produces a typical withdrawal abstinence syndrome (Table 7-3).

VI. Opioid Agonists (see Table 7-1). The most notable feature of the clinical use of opioids is the extraordinary variation in dose requirements for effective treatment of pain.
A. Morphine is the prototype opioid agonist to which all other opioids are compared, producing analgesia, euphoria, sedation, nausea, and pruritus (especially in the cutaneous areas around the nose) (see Fig. 7-1). The cause of pain persists, but even low doses of morphine increase the threshold to pain and modify the perception of noxious stimulation such that it is no longer experienced as pain (continuous, dull pain is relieved by morphine more effectively than is sharp, intermittent pain). In contrast to nonopioid analgesics, morphine is effective against pain arising from the viscera. Analgesia is most prominent when morphine is administered before the painful stimulus occurs (in the absence of pain, however, morphine may produce dysphoria rather than euphoria).
1. Pharmacokinetics
a. Morphine is well absorbed after intramuscular (IM) administration, with onset of effect in 15 to 30 minutes, a peak effect in 45 to 90 minutes and a clinical duration about 4 hours.
b. Morphine is usually administered IV in the perioperative period, thus eliminating the unpredictable influence of drug absorption. The peak effect (equilibration time between the blood and brain) after IV administration of morphine is delayed compared with opioids such as fentanyl and alfentanil, requiring about 15 to 30 minutes (Table 7-4).
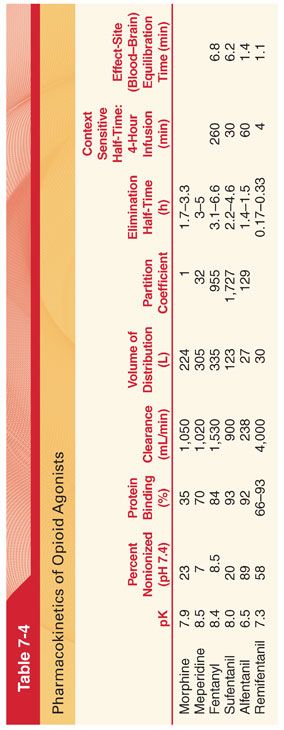
c. Only a small amount of administered morphine gains access to the central nervous system (CNS) (estimated that <0.1% of morphine that is administered IV has entered the CNS at the time of peak plasma concentrations).
2. Metabolism of morphine is primarily through conjugation with glucuronic acid in hepatic and extrahepatic sites, especially the kidneys (about 75% to 85% of a dose of morphine appears as morphine-3-glucuronide [pharmacologically inactive], and 5% to 10% as morphine-6-glucuronide [pharmacologically active with an analgesic potency 650-fold higher than morphine]).
3. Elimination Half-Time (see Table 7-4). The decrease in the plasma concentration of morphine after initial distribution of the drug is principally due to metabolism because only a small amount of unchanged opioid is excreted in the urine. Concentrations of morphine in the colostrum of parturients receiving patient-controlled analgesia with morphine are low and it is unlikely that significant amounts of drug will be transferred to the breast-fed neonate.
B. Meperidine is a synthetic opioid agonist at μ and κ opioid receptors (analogues of meperidine include fentanyl, sufentanil, alfentanil, and remifentanil). Meperidine shares several structural features that are present in local anesthetics and structurally is similar to atropine (possesses a mild atropine-like antispasmodic effect on smooth muscle).
1. Pharmacokinetics. Meperidine is about one-tenth as potent as morphine producing equivalent sedation, euphoria, nausea, vomiting, and depression of ventilation with a duration of action of 2 to 4 hours.
2. Metabolism is extensive, with about 90% of the drug initially undergoing demethylation to normeperidine and hydrolysis to meperidinic acid. Normeperidine (one-half as active as an analgesic) subsequently undergoes hydrolysis to normeperidinic acid. Normeperidine toxicity manifesting as myoclonus and seizures is most likely during prolonged administration (3 days) of meperidine as during patient-controlled analgesia, especially in the presence of impaired renal function.
3. Elimination half-time of meperidine is 3 to 5 hours (see Table 7-4).
4. Clinical Uses
a. The clinical use of meperidine has declined greatly in recent years patient-controlled analgesia with meperidine cannot be recommended because of possible normeperidine toxicity.
b. Meperidine may be effective in suppressing postoperative shivering that may result in detrimental increases in metabolic oxygen consumption (antishivering effects may reflect stimulation of κ receptors)
5. Side effects of meperidine generally resemble those described for morphine, but meperidine, in contrast to morphine, rarely causes bradycardia but instead may increase heart rate, reflecting its modest atropine-like qualities. Large doses of meperidine result in decreases in myocardial contractility, which, among opioids, is unique for this drug.
C. Fentanyl is a phenylpiperidine-derivative synthetic opioid agonist that is structurally related to meperidine. As an analgesic, fentanyl is 75 to 125 times more potent than morphine.
1. Pharmacokinetics
a. A single dose of fentanyl administered IV has a more rapid onset and shorter duration of action than morphine. Despite the clinical impression that fentanyl produces a rapid onset, there is a distinct time lag between the peak plasma fentanyl concentration and peak slowing on the electroencephalogram (EEG) (reflects the effect-site equilibration time between blood and the brain for fentanyl, which is 6.4 minutes). The greater potency and more rapid onset of action reflect the across the blood–brain barrier.
b. The lungs also serve as a large inactive storage site, with an estimated 75% of the initial fentanyl dose undergoing first-pass pulmonary uptake. (This nonrespiratory function of the lungs limits the initial amount of drug that reaches the systemic circulation and may play an important role in determining the pharmacokinetic profile of fentanyl.)
2. Metabolism. Fentanyl is extensively metabolized by N-demethylation and the pharmacologic activity of fentanyl metabolites is believed to be minimal.
3. Elimination Half-Time
a. Despite the clinical impression that fentanyl has a short duration of action, its elimination half-time is longer than that for morphine (see Table 7-4). This longer elimination half-time reflects a larger volume of distribution (Vd) of fentanyl due to its greater lipid solubility and thus more rapid passage into highly vascular tissues compared with the less lipid-soluble morphine (more than 80% of the injected dose leaves the plasma in <5 minutes).
b. The plasma concentrations of fentanyl are maintained by slow reuptake from inactive tissue sites, which accounts for persistent drug effects that parallel the prolonged elimination half-time.
c. A prolonged elimination half-time for fentanyl in elderly patients is due to decreased clearance of the opioid because Vd is not changed in comparison with younger adults.
4. Context-Sensitive Half-Time. As the duration of continuous infusion of fentanyl increases beyond about 2 hours, the context-sensitive half-time of this opioid becomes greater than sufentanil (Fig. 7-3). This reflects saturation of inactive tissue sites with fentanyl during prolonged infusions and return of the opioid from peripheral compartments to the plasma. This tissue reservoir of fentanyl replaces fentanyl eliminated by hepatic metabolism so as to slow the rate of decrease in the plasma concentration of fentanyl when the infusion is discontinued.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


