Open Gastric Bypass for Morbid Obesity
Ronald H. Clements
Introduction
Morbid obesity continues to be a major nutritional health crisis for most of the world despite the relatively recent exponential growth in bariatric surgery that is largely due the application of laparoscopic techniques. The widely accepted definitions and recommendations from the National Institutes of Health Consensus statement of 1991 continue to guide surgical treatment. Patients with body mass index (BMI; kg/m2) over 40 or those with BMI over 35 with significant medical comorbidities should be considered for operative intervention if they are acceptable candidates after an extensive multidisciplinary evaluation. Most elective bariatric cases are begun with the intention of completing them with minimally invasive techniques, but the bariatric surgeon must have the requisite skill and experience to convert immediately to the open technique when necessary. Additionally, many revisional procedures are done as planned open cases due to the increased incidence of intra-abdominal adhesions and to regain the advantage of tactile sensation. The open gastric bypass is still an acceptable primary procedure for the surgeon who does not possess the advanced minimally invasive skills necessary to perform the operation laparoscopically. The technical description in this chapter applies equally well to the elective open, revisional, or converted gastric bypass.
Technique
The patient is placed on the operating table in the supine position and secured appropriately. A large footboard is attached to the table to prevent the patient from sliding
downward when the table is placed into steep, reversed Trendelenburg position. Additionally, a wide padded strap is placed across the patient’s thighs to prevent flexion of the knees when this position is assumed. Both arms are extended on padded arm boards, and secured by wrapping them with gauze to prevent them from falling when the patient is placed into steep, reversed Trendelenburg position.
downward when the table is placed into steep, reversed Trendelenburg position. Additionally, a wide padded strap is placed across the patient’s thighs to prevent flexion of the knees when this position is assumed. Both arms are extended on padded arm boards, and secured by wrapping them with gauze to prevent them from falling when the patient is placed into steep, reversed Trendelenburg position.
After general anesthesia is induced, appropriate prophylactic antibiotics are infused, a Foley catheter is inserted, and antithrombotic measures are employed, an upper midline incision is made extending downward about 15 to 20 cm from the xiphoid. The adipose tissue is fractured bluntly down to the linea alba, and minor bleeding is easily controlled with electrocautery. The fascia is incised in the midline, and the peritoneum is entered under direct vision taking care to avoid visceral injury if there is suspicion of intra-abdominal adhesions. The abdomen is explored in a systematic fashion to exclude unexpected pathology. As a matter of routine, a core needle biopsy is taken from the left lateral segment of the liver at this stage of the procedure in order to avoid false interpretation of inflammation that may be induced by retraction. This biopsy is done because neither preoperative blood test nor intraoperative liver appearance can predict the presence or severity of nonalcoholic steatohepatitis (NASH) or fibrosis.
After abdominal exploration and liver biopsy, creation of the Roux limb is accomplished by first exposing the ligament of Treitz. This is expeditiously done in the deep, fat-filled abdomen by lifting the omentum and the attached transverse colon from the abdominal cavity and retracting them superiorly with both hands by the assistant. This maneuver allows the transverse mesocolon to function as a curtain that keeps all the upper abdominal contents from falling down over the origin of the jejunum. The multiple loops of small bowel are retracted caudally, and the ligament of Treitz with its accompanying inferior mesenteric vein is exposed. At a point approximately 30 to 40 cm from the ligament of Treitz the jejunum is transected with a stapling device. The mesentery is divided parallel to the radial arcade of vessels in order to avoid undercutting the bowel, which could compromise the blood supply of either the Roux limb or the biliopancreatic limb. This mesenteric division can provide additional length to the jejunum allowing it to reach the gastric pouch without tension. An absorbable suture is placed on the distal side (Roux limb) of this transected bowel in order to avoid connecting the biliopancreatic limb to the gastric pouch later in the operation (Roux-en-“O”). The Roux limb length is measured according to the surgeon’s preference (95 cm if BMI <50 or 150 cm if BMI ≥50), and the biliopancreatic limb is anastomosed to the jejunum at this location in a side-to-side fashion using stapling devices. The mesenteric defect is closed with a nonabsorbable suture placed in figures of eight. With the Roux-en-Y now complete, the jejunum is returned to the abdominal cavity, and attention is turned to the creation of the gastric pouch.
The use of a sturdy retractor system that firmly attaches to the operating table is strongly recommended. Lighter weight retractors or those that attach to the table at only one point tend to shift or twist under the strain of the heavy abdominal wall associated with this population. Adequate exposure is the key hurdle to overcome in the safe and efficient conduct of the open gastric bypass. This is especially important if the case is converted from the laparoscopic technique because adverse conditions (extremely thick abdominal wall, hepatomegaly, bleeding, anastomotic leaking, etc.) necessitate opening the abdomen. Also, if the patient is supermorbidly obese the operative site can be quite deep in the open abdomen, making exposure challenging. The author prefers the Gomez (Pilling Weck Surgical, Fort Washington, PA) retractor system because it attaches to the operating table at four points. A square frame to which each retractor attaches is supported by these four posts (Fig. 1). This retractor system also ensures the patient doesn’t shift on the operating table in the extremes of reverse Trendelenburg or lateral tilting. Two large body wall retractors are used to elevate and retract both costal margins, and a “fingers” retractor is used to elevate and retract the left lateral segment of the liver. If needed for additional exposure the falciform ligament can be divided to allow more retraction toward the patient’s right.
The patient is now placed into the full reversed Trendelenburg position to adequately expose the gastroesophageal junction. A 34 French gastric lavage tube is advanced into the stomach and is palpated at the left crus of the diaphragm (Fig. 2A). The visceral peritoneum reflecting off of the greater curvature of the stomach onto the diaphragm at the angle of His is divided bluntly with a finger of the surgeon’s right hand pointing inferiorly and medially under the tube positioned in the stomach.
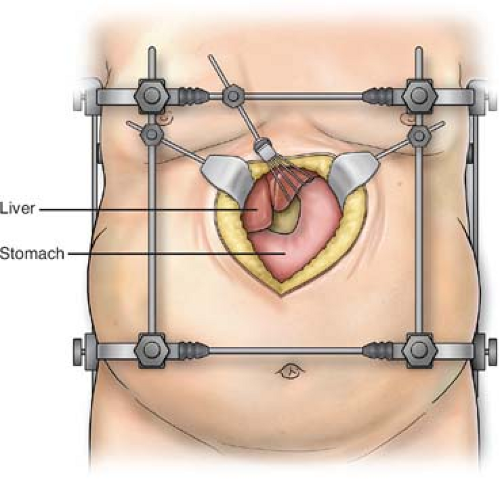 Fig. 1. Placement of the retractor system in the upper midline incision to expose the gastroesophageal junction to facilitate construction of the gastric pouch. |
The avascular portion of the gastrohepatic ligament overlying the caudate lobe of the liver is opened bluntly. This allows placement of the surgeon’s left hand posterior to the lesser curve of the stomach aiming toward the angle of His. This should allow the middle finger of the left hand to meet the middle finger of the right hand that was placed in this same plane from the greater curvature, thus creating a retrogastric tunnel (Fig. 2B). If downward traction is needed, a Penrose drain can be inserted from the dissection point at the angle of His, passed behind the stomach, and brought through the opening in the gastrohepatic ligament. At a point approximately
6 to 8 cm from the gastroesophageal junction, the small vascular branches of the left gastric artery supplying the lesser curve of the stomach are divided to expose the lesser curve while preserving the left gastric pedicle and the vagus nerves. If the Penrose was placed, the medial end is brought through this opening, thus preventing the left gastric artery from being divided as the stomach is transected with the stapling device. The Penrose drain is helpful to facilitate the passage of the device if the surgeon chooses to use a 100-mm linear stapling device to transect the stomach with one firing. While it is convenient to staple only once, exposing this can be problematic if the patient has a bulky liver or a large amount of fat at the esophagogastric junction. The author’s current preference is to use the articulating, laparoscopic linear stapling device because it has a long, narrow shaft that is easily rotated out of the surgeon’s line of sight. The articulating stapler also enables construction of a more vertically oriented, lesser curve-based, tubular gastric pouch. This is important to prevent pouch dilation over time and to provide thicker, more muscular tissue for the gastrojejunostomy later in the operation. Usually three firings of the laparoscopic stapler (one 45 mm transversely oriented and two 60 mm vertically oriented alongside the gastric lavage tube) are needed to completely divide the stomach. At this level on the stomach, the blue cartridges provide adequate staple height to completely penetrate the tissue and ensure hemostasis of the staple line. No buttressing material or oversewing of the staple line is necessary.
6 to 8 cm from the gastroesophageal junction, the small vascular branches of the left gastric artery supplying the lesser curve of the stomach are divided to expose the lesser curve while preserving the left gastric pedicle and the vagus nerves. If the Penrose was placed, the medial end is brought through this opening, thus preventing the left gastric artery from being divided as the stomach is transected with the stapling device. The Penrose drain is helpful to facilitate the passage of the device if the surgeon chooses to use a 100-mm linear stapling device to transect the stomach with one firing. While it is convenient to staple only once, exposing this can be problematic if the patient has a bulky liver or a large amount of fat at the esophagogastric junction. The author’s current preference is to use the articulating, laparoscopic linear stapling device because it has a long, narrow shaft that is easily rotated out of the surgeon’s line of sight. The articulating stapler also enables construction of a more vertically oriented, lesser curve-based, tubular gastric pouch. This is important to prevent pouch dilation over time and to provide thicker, more muscular tissue for the gastrojejunostomy later in the operation. Usually three firings of the laparoscopic stapler (one 45 mm transversely oriented and two 60 mm vertically oriented alongside the gastric lavage tube) are needed to completely divide the stomach. At this level on the stomach, the blue cartridges provide adequate staple height to completely penetrate the tissue and ensure hemostasis of the staple line. No buttressing material or oversewing of the staple line is necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


