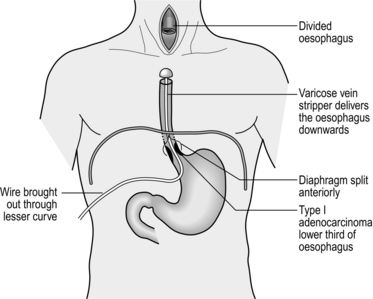
8 1. Endoscope every patient with dysphagia except when this is fully explained by the presence of neurological or neuromuscular disease. 2. Endoscope patients with suspected disease in the oesophagus producing pain on swallowing (odynophagia), heartburn not responding to simple medication or arising de novo in patients over 50 years, bleeding, or if accidental and iatrogenic damage are suspected. 1. Ensure that the endoscope, the ancillary equipment and necessary spares are available, function correctly and are appropriately sterile. The endoscope must be thoroughly prepared between procedures according to the maker’s instructions. Fibreoptic instruments, biopsy forceps and similar instruments are scrupulously cleaned using neutral detergent and usually disinfected with 2% alkaline glutaraldehyde. This is capable of eliminating all infective organisms, including HIV. Washing and sterilization are performed mechanically in an automatic machine to avoid exposure of endoscopy room staff to glutaraldehyde fumes. 2. Modern gastrointestinal endoscopes are slim, versatile, have remarkably flexible tips and can be passed with pharyngeal anaesthesia alone in most patients. They are safe, relatively comfortable for the patient and allow examination of the stomach and duodenum beyond. Use the end-viewing instrument routinely since it gives the best general view. Through it can be passed biopsy forceps, cytology brushes, snares, guidewires for dilators and needles for injection. Argon plasma coagulation or Nd-YAG laser may be applied through it for the palliation of inoperable neoplasms or for the treatment of Barrett’s oesophagus. The technology of endoscopes is steadily improving and the rigid oesophagoscope is, to all intents and purposes, obsolete. 3. Obtain signed informed consent from the patient. 4. Remove dentures from the patient. 5. Except in an emergency, have the patient starved of food and fluids for at least 5 hours. In an emergency, especially in patients with upper gastrointestinal haemorrhage who cannot wait 5 hours for the stomach to empty, a crash general anaesthetic with cricoid pressure is the safest means of securing the airway and preventing aspiration. 6. Obtain a preliminary barium swallow X-ray if there is a suspected pharyngeal pouch. 7. Attach a pulse oximeter probe to the patient’s finger if sedation is being used, and ensure that there are sufficient numbers of staff in the endoscopy room for safe care of a sedated patient. 8. Spray the pharynx with lidocaine solution just before passing the endoscope. 9. In anxious patients, or those in whom intervention (e.g. dilatation) is required, insert a small plastic cannula into a peripheral vein and through it inject slowly 1–2 mg of midazolam until the patient’s eyelids just begin to droop. Remember that it takes 2 minutes for the full effect of midazolam to develop. 1. Lay the patient on the left side with hips and knees flexed. Place a plastic hollow gag between the teeth. Ensure that the patient’s head is in the midline and that the chin is lowered on to the chest. 2. Lubricate the previously checked end-viewing instrument with water-soluble jelly. 3. Pass the endoscope tip through the plastic gag, over the tongue to the posterior pharyngeal wall. Depress the tip control slightly so that the instrument tip passes down towards the cricopharyngeal sphincter. Do not overflex the tip or it will be directed anteriorly and enter the larynx. Visualize the larynx and pass the endoscope just behind it. 4. Ask the patient to swallow. Do not resist the slight extrusion of the endoscope as the larynx rises, but maintain gentle pressure so that it will advance as the larynx descends and the cricopharyngeal sphincter relaxes. Advance the endoscope under vision, insufflating air gently to open up the passage. Aspirate any fluid. Spray water across the lens if it becomes obscured. If no holdup is encountered, pass the tip through the stomach into the duodenum then withdraw it slowly, noting the features. Remove biopsy specimens and take cytology brushings from any ulcers, tumours or other lesions. 5. If a stricture is encountered note its distance from the incisor teeth. Sometimes the instrument will pass through, allowing the length of the stricture to be determined. Always remove biopsy specimens and cytology brushings from within the stricture. If the stricture is benign in appearance, gentle dilatation to 12 mm can be attempted if the patient is symptomatic. Dilatation of malignant strictures is not indicated as any benefit is short-lasting and the risk of perforation is high (6–8%). Get biopsies and confirm the diagnosis prior to intervention. If nutritional support is required, fluoroscopic passage of a feeding nasogastric tube can be performed. 1. Note the level of each feature. The cricopharyngeal sphincter is approximately 16 cm from the incisor teeth. The deviation around the aortic arch is 28–30 cm, the cardia lies at 40 cm and here the lining changes abruptly from the pale, bluish, stratified oesophageal epithelium to the florid, pinker, gastric columnar-cell epithelium. 2. Oesophagitis is usually from gastro-oesophageal reflux, but is not necessarily associated with hiatal hernia. Consult a colour chart that illustrates the grades of oesophagitis. Most commonly there are red streaking erosions just above the cardia. Oesophagitis may be seen above a benign stricture. Occasionally, in advanced achalasia, one may see a mild diffuse oesophagitis from contact with fermenting food residues. Thick white plaques indicate monilial infection, usually in association with oral involvement. Confirm the diagnosis by taking mucosal scrapings. 3. Sliding hiatal hernia produces a loculus of stomach above the constriction of the crura with a raised gastro-oesophageal mucosal junction. To determine the level of the hiatus, ask the patient to sniff, and note the level at which the crura momentarily narrow the lumen. Reflux and oesophagitis may be visible. A rolling hernia is visible only from within the stomach by inverting the tip of a flexible instrument to view the apparent fundic diverticulum. If the diagnosis is a possibility, confirm with a barium study. 4. Frank ulceration in the oesophagus is unusual, but may be due to severe reflux disease. In Barrett’s oesophagus the lower gullet is lined with modified gastric mucosa and an ulcer may develop in the columnar-lined segment. In all cases of Barrett’s take biopsies of the columnar segment from all four quadrants at 2-cm intervals. In patients with dysplasia even more biopsies are required for accurate assessment. Use ‘jumbo’ forceps. Ulcerating carcinomas may develop at any level. In most Western countries the majority of cancers comprise adenocarcinomas and these arise in the lower oesophagus in association with Barrett’s oesophagus. Take multiple biopsies and cytological brushings from a number of areas of all ulcers. 5. Strictures from peptic oesophagitis or, rarely, ulceration in a Barrett’s oesophagus develop at any time from birth onwards, but more frequently occur in middle or old age. Almost always there is a coincidental hiatal hernia. If there is no hernia below the stricture, suspect cancer. Also suspect cancer if there is food residue above a stricture. Food residue may also be seen in achalasia and may be the only diagnostic clue. Take multiple biopsies and brushings for cytology. The cause of Schatzki’s ring is unknown. It is usually asymptomatic, seen radiologically at the junction between gastric and oesophageal mucosa. Caustic strictures develop at the sites of hold-up of swallowed liquids at the cricopharyngeus, at the aortic arch crossing and at the cardia. Webs or strictures in the upper oesophagus are uncommon. However, it is not unusual to see a patch or ring of ectopic gastric mucosa in the upper oesophagus 1–2 cm below the cricopharyngeus, the so-called ‘inlet patch’. Stricture may arise from external pressure, of which by far the most common cause is bronchogenic carcinoma. 6. Mega-oesophagus may be seen in achalasia of the cardia, but is now uncommon as most cases are diagnosed long before dilatation takes place. Mega-oesophagus may also be seen in the South American Chagas’ disease and in some cases of advanced scleroderma. 7. Pulsion diverticula are related to abnormal oesophageal motility and are seen above the cricopharyngeus muscle (Zenker’s diverticulum or pharyngeal pouch) and above segments of presumed spasm. Traction diverticula in the mid-oesophagus develop as a result of chronic inflammation of mediastinal glands, especially from tuberculosis. 8. Oesophageal varices are usually recognized just above the cardia as convoluted varicose veins, which may extend into the upper stomach. 1. Fragile strictures do not always require endoscopic dilatation if the diagnosis is not in doubt or has been confirmed by endoscopy. The safest oesophageal dilator is soft, solid food, provided that each bolus contains only aggregated small particles. 2. Record the distance of the stricture from the incisor teeth. If the stricture is short and appears benign, the best means for dilatation is by a through-the-channel balloon. These balloons have a fixed maximum diameter of 2 cm (60 F). Always check for perforation with endoscopy after dilatation. An alternative to these balloon dilatators are soft mercury-laden Maloney dilators. If problems occur inserting them through a tortuous stricture, balloon dilatation using a hydrophilic guidewire inserted under fluoroscopic control should be undertaken. 3. First outline the passage by getting the erect patient to swallow a thin contrast medium. Now introduce a fine, flexible guidewire through a nostril into the oesophagus and negotiate the tip through the stricture using a combination of rotating it and getting the patient to swallow a little water or more contrast medium to outline the passage. Now pass a well-lubricated catheter fitted with a deflated balloon over the guidewire and insinuate it through the stricture. The proximal and distal ends of the balloon have radio-opaque markers so that it can be placed accurately. Carefully inflate the balloon, watching it on the screen. A waist appears at the level of the stricture and as the pressure is gradually increased this disappears. If the patient is apprehensive or complains of discomfort, temporarily stop inflating the balloon. 4. Most strictures can be dilated at a single session, but do not persist unduly in the face of difficulty or discomfort. When the balloon is withdrawn, check to see if there is any blood on it. Now give the patient some contrast medium to swallow and carefully watch it pass through the stricture to ensure that there is no leak. 1. Swallowed articles impact at the sites of narrowing. Objects at the cricopharyngeus muscle are regurgitated but those that pass this point may impact at the crossing of the aortic arch or at the cardia. However, the normal oesophagus is extremely distensible and smooth objects usually pass into the stomach. The most frequent causes of impaction are pre-existing stricture or a sharp foreign body that penetrates the oesophageal wall. 2. Remember that many impacted foreign bodies are radiolucent. Sometimes they are demonstrable on X-rays by giving the patient a drink of water-soluble contrast medium. 3. Most foreign bodies may be removed using a variety of methods in conjunction with fibreoptic endoscopes and an overtube under local anaesthesia with sedation. If a foreign body cannot be removed with modern endoscopic instruments an operation is probably required. Deeply and firmly impacted foreign bodies may require thoracotomy and oesophagotomy to remove them. 4. A smooth foreign body, or a food bolus, may be gently pushed into the stomach. As a rule it will pass through the gut but if it remains in the stomach removal is easier than from the oesophagus. 1. There is a classic repertoire of methods to remove foreign bodies through the rigid oesophagoscope. The grasping forceps are strong and versatile, and can cope with open safety pins and coins. Version and extraction of an open safety pin with the point facing upwards is now part of the folklore of oesophageal surgery. However, the use of the rigid endoscope is not now to be encouraged. There are safer and better methods. 2. Ingenious methods have been used to remove foreign bodies using the end-viewing fibreoptic endoscope. The foreign body may be grasped with forceps or caught with a snare and withdrawn together with the instrument. An external flexible sheath may be pushed over the end of the endoscope tip into which a sharp foreign body can be drawn to protect the mucosa from injury. A variety of snares and grasping forceps should be kept available. 1. Recognize these as soft, collapsible, projecting columns in the lower oesophagus, sometimes continuing into the gastric cardia. 2. In patients with upper gastrointestinal bleeding who are found to have varices, do not assume that the bleeding is from the varices. A high proportion of such patients have another cause such as peptic ulcer, so always carry out a complete examination. 3. The varices thrombose when injected with ethanolamine oleate warmed to reduce its viscosity. An injection needle is passed down the biopsy channel and 2–5 ml is injected into each varix. The injections are repeated at intervals of 3–4 weeks until the varices are obliterated. 4. Banding of varices has a number of advantages, including better immediate control of active bleeding. A banding device that can fire several bands is loaded into and onto the endoscope. Always read the instructions on the device. Continuous design improvement is the norm and the device may have changed since you last used it. The varix is identified and then sucked into the banding cap on the end of the endoscope. When a ‘red-out’ is achieved the bander is fired and the suction released. The band should be seen clearly in the required position. Multiple varices can be ligated at a single session. 1. It is not necessary to intubate all strictures that cannot be resected. Malignant strictures that will be submitted to chemo-or radiotherapy may deteriorate temporarily and later expand, so it is worthwhile deferring intubation. Intubation of benign strictures is not recommended unless in exceptional cases in very frail subjects with a limited life-expectancy. Intubation produces a rigid channel through which food must fall by gravity and which can easily become blocked, so reserve its use for patients with real need. 2. The development of expanding metal stents is a considerable advance and has replaced the rigid plastic tube. They provide a larger lumen for swallowing and do not require as much dilatation as semirigid stents such as the Nottingham tube. As a result insertion is safer. Expanding metal stents may be inserted at endoscopy, by radiological screening or by a combination of both methods. They are produced in both covered and uncovered formats. 1. There are many different designs. Be familiar with the stent, its introducer and the method of deployment. Most expanding stents shorten as they expand and this must be taken into account during insertion. Some stents can be partially deployed and the position adjusted if it is not satisfactory. However, you may have to get it right first time, so be careful. 2. Such stents can be inserted under fluoroscopic or endoscopic control. Fluoroscopy has the advantage of outlining the length and position of the stricture accurately by using a radio-opaque contrast swallow prior to insertion. Dilatation up to 1 cm is usually required prior to stent deployment, as described above for dilatation of difficult strictures. 3. Pass the stent with its introducer over the guidewire and into the desired position. An endoscope may be passed alongside the guidewire so that deployment can be checked under vision. 4. Deploy the stent according to the manufacturer’s instructions. 5. Correct stent deployment can be checked by endoscopy or contrast swallow. Some stents may be very slow to expand. Expansion may be hastened by dilatation with a through-the-scope balloon. 1. Make sure the patient does not have chest pain, air emphysema in the neck, or a raised temperature. Have a plain chest radiograph taken or perform a contrast swallow. 2. If there is evidence of a leak, confirm it and identify the site with X-rays using a water-soluble contrast medium. If an expanding stent is not sealing a leak consider inserting a second stent. Start the patient on broad-spectrum antibiotics and withhold food and fluids until the patient is entirely comfortable and a contrast swallow shows no leak. 3. Following stent insertion, warn the patient against swallowing unchewed food, particularly lumps of meat, fruit skins and stones, and to wash down the food with sips of water. Aerated drinks such as sodium bicarbonate solution (half a teaspoonful in half a glass of water half an hour before meals) or fresh pineapple juice help to wash away adherent mucus that may block the tube. 4. It is now extraordinarily uncommon to fail to intubate a tumour. If it cannot be done, a feeding gastrostomy or jejunostomy may be inserted after full discussion with the patient. This poses ethical and philosophical dilemmas, but these must be faced. However, always remember that the aim of palliation is to improve the quality of remaining life. If a particular therapy will not improve the quality of life in an individual patient, do not use it. 1. The cervical oesophagus may be approached from either side. Operations for the removal of pharyngeal pouch and cricopharyngeal myotomy, are usually carried out from the left side. For oesophageal anastomosis following resection, either side can be used. The right-sided approach minimizes risk of damage to the thoracic duct, although this is a rare complication for exposure of the oesophagus and usually occurs as a complication of biopsy of lymph nodes. The left recurrent laryngeal nerve is more likely to be injured during intrathoracic resection or be involved in the malignant process. It is better, therefore, to expose it to risk of injury rather than the right nerve. 2. The anaesthetized intubated patient lies supine on the operating table with the head turned to the opposite side from which the exploration will be made, resting on a ring with the neck extended. There is no need for complex head towelling, but drapes can be secured with skin staples. 3. Incise along the anterior border of sternomastoid muscle, through platysma muscle, cervical fascia, omohyoid muscle, ligating and dividing the middle thyroid vein to enter the space between the oesophagus, trachea and thyroid gland medially and the carotid sheath laterally. The inferior thyroid artery crosses the space; ligate and divide it laterally only if it interferes with the dissection. 4. Rotate the whole oesophageal-tracheal-thyroid column towards the opposite side, bringing into view the trachea-oesophageal groove, and display the posterior surface of the oesophagus and lower pharynx. Beware of inserting a Langenbeck’s retractor into the trachea-oesophageal groove since it may well crush the recurrent laryngeal nerve. 5. Mobilize the posterior wall of the oesophagus with blunt dissection. Staying on the muscle wall of the oesophagus, come anteriorly and over the front, separating the trachea and recurrent laryngeal nerve anteriorly (Fig. 8.4). It is not necessary to mobilize the nerve and this prevents an ischaemic neuropraxia. Staying on the muscle wall, go round the oesophagus on the opposite side, retracting the oesophagus laterally. This exposes the prevertebral fascia on the opposite side. A curved forceps can then be placed around the oesophagus and a sling placed. With gentle traction on this, the oesophagus can be mobilized by finger dissection, staying on the oesophageal wall. 1. The anaesthetized patient, intubated with a double-lumen tube to allow exclusion of the right lung, lies on the left side. Carry out right posterolateral thoracotomy at the level of the fifth or sixth rib. 2. Ask the anaesthetist to collapse the right lung. Draw it downwards and forwards to reveal the mediastinal pleura. The oesophagus cannot be seen but the azygos vein can be seen arching over the lung root. Incise the mediastinal pleura, mobilize, doubly ligate and divide the azygos vein. This reveals the oesophagus running posterior to the trachea and lung root. The lower oesophagus is not visible between the left atrium and the vertebral column as it veers to the left. Expose it by dividing the pulmonary ligament until the inferior pulmonary vein is exposed. Then divide the mediastinal pleura anterior to the descending aorta. The upper stomach can be approached after dilating or incising the diaphragmatic crus to enlarge the hiatus. 1. The lower thoracic oesophagus and upper stomach are best approached using a combined thoracoabdominal approach. 2. Lay the anaesthetized intubated patient on the right side, left leg extended, right leg flexed at hip and knee, both arms flexed with forearms before the face. Allow the patient to lie back with the shoulders at 30° from the vertical. Fix the patient’s hips with an encircling band; support the left upper scapula against a padded post. 3. Prepare the skin and drape the area with sterile towels. 4. Start the incision 2.5 cm under the right costal margin in the midclavicular line, carry it obliquely upwards and to the left to cross the costal margin along the line of the seventh or eighth rib, extending to the posterior angle of the chosen rib and up behind the scapula. Deepen the incision to enter the thorax along the line of the rib, cutting and removing 1 cm of the costal margin. Incise the diaphragm peripherally parallel to the chest wall far enough in to enable the chest to be opened but not as far as the hiatus. This method spares the phrenic nerve. 5. In case of doubt make the abdominal or thoracic part of the incision first; assess the condition and now, if indicated, extend it fully. 6. After completing the procedure, close the diaphragm with strong absorbable material. Do not suture the costal margin but resect a wedge of cartilage to enable the ends to lie adjacent. Suture the abdomen in the usual manner. Close the chest after inserting an underwater-sealed drain. 1. The lower oesophagus is approachable through the abdomen and oesophageal hiatus. 2. The best access, especially in patients with a wide costal angle, is by a roof-top or bilateral subcostal incision. In those with a narrow costal angle an upper midline incision extending to the costal margin is preferable, opening the peritoneum just to the left of the falciform ligament. Ligate the ligamentum teres. Divide it and the falciform ligament. 3. Draw down the stomach while an assistant elevates the left lobe of the liver with a flat-bladed retractor. The lower oesophagus can be felt at the hiatus. 4. If necessary, cut the left triangular ligament and fold the left lobe of the liver to the right. 5. To display the lower thoracic oesophagus, transversely incise the peritoneum and fascia over the abdominal oesophagus for 5 cm, preserving the anterior vagal trunk. If greater exposure is necessary, insert a finger into the posterior mediastinum. Turn it forwards to separate the pericardium from the upper surface and incise the crus and diaphragm anteriorly for 5–7 cm. In patients of suitable build, the oesophagus can be viewed almost up to the carina of the trachea if the heart is gently elevated with a flat retractor. It is usually unnecessary to close the incision in the crus and diaphragm. The inferior phrenic vein will need to be oversewn. 1. This encompasses both the abdominal and cervical approaches described above. 2. After division of the diaphragm anteriorly, dissect the fat pad off the back of the pericardium. It is common to take the pleura on the left side. Place an anterior resection retractor behind the heart and lift up and towards the head. Take care not to compress the left atrium. Dissect under direct vision to the carina using a harmonic scalpel, dividing the vagi. 3. Mobilize the oesophagus in the neck as described from the left side. After encircling the oesophagus, mobilize it with a finger into the upper mediastinum. 4. Place a tie around the oesophagus low down and produce a mucosal tube as described below. Open the anterior part of the mucosal tube and withdraw the nasogastric tube. Grasp the end of the nasogastric tube and place a tie into it to facilitate bringing it back to the conduit during the anastomosis. 5. Pass the wire of a varicose-vein stripper into the oesophagus and pass it into the stomach. Tighten the tie round the stripper wire distal to its insertion and divide the oesophagus. Place a medium head on the stripper. Gently pull the stripper distally, guiding the head into the posterior mediastinum avoiding damage to the adjacent structures. This will invert the oesophagus and deliver it to the abdomen. Vagal strands may prevent full delivery and need division under direct vision from below. 6. To guide the conduit to the neck for anastomosis use a chest drain to avoid rotation. 1. Position the patient supine with the legs flat (for diagnostic or staging endoscopy) or with the legs apart in stirrups, as in rectal surgery (for major procedures, such as antireflux surgery). 2. Induce a pneumoperitoneum using a Veress needle or by an open technique. 3. Insert a 10-mm cannula in the midline approximately one-third of the distance between the umbilicus and the xiphisternum. 4. Liver retraction is best achieved by use of a Nathanson retractor inserted via a small 5-mm incision in the epigastrium. If not available, insert a 5- or 10-mm cannula, depending on what type of liver retractor is used, in the right subcostal region in the midclavicular line. Introduce a liver retractor and lift the left lobe of the liver upwards. 5. The number and position of additional cannulas depend on the procedure to be done and the preference of the operator. Commonly, additional cannulas are placed below the xiphisternum, in the left subcostal region in the midclavicular line and the anterior axillary line (the ‘Liege’ approach). 6. The oesophagus may then be approached as described in the previous section by dividing the peritoneum over the lower oesophagus. The precise approach to the oesophagus depends on the procedure to be done. 1. Most other parts of the bowel are covered with serosa which rapidly forms fibrinous adhesions, sealing small defects and preventing leaks. The oesophagus has no serous coat except on the anterior wall of the abdominal segment. 2. A considerable part of the oesophageal wall is composed of longitudinal muscle. Longitudinally placed sutures thus have a tendency to cut out. The powerful longitudinal muscle produces shortening of the transected oesophagus when it contracts. Unless this is allowed for, the most carefully placed sutures may be torn out. 3. When the oesophagus is completely relaxed it has a remarkably large lumen. Commonly, the action of the circular muscle makes the diameter appear to be small. However, it can be stretched quite easily by insertion of a Foley catheter and inflating the balloon to facilitate the placement of sutures. If this is not done, closely spaced sutures may become widely separated on stretching, and leakage can easily occur between them. 4. The blood supply to the oesophagus is tenuous when it is mobilized, especially at the lower end. 5. The healthy oesophagus is easily damaged but disease may make it exceptionally fragile. 6. A diseased or partially obstructed oesophagus is contaminated. Prophylactic antibiotics must be given to cover the operation. 7. Although oral feeding may be stopped temporarily following oesophageal surgery, swallowed saliva must still pass through. 8. Intrathoracic oesophageal leakage produces posterior mediastinitis and if the pleura is damaged a pleural collection develops. The best hope for the patient’s survival if major leakage occurs is rapid clinical recognition with early re-operative repair and drainage. Minor leaks may be treated conservatively. Place chest drains in all opened chest cavities. Following transhiatal resection, even if the pleura is not opened a chest drain in the posterior mediastinum will prevent haematoma collection. A feeding jejunostomy should be placed at operation in all cases for postoperative nutrition and oral intake should not commence until the anastomosis is checked by contrast swallow at 5 days. 1. The tenuous blood supply of the oesophagus makes it rarely possible to excise a segment, mobilize the cut ends and carry out an end-to-end union, except in neonates. Anastomosis is therefore usually to stomach, jejunum or colon. 2. Sutured anastomoses have been described using many different methods and materials. It is now recognized that single-layer anastomoses with continuous or interrupted sutures are best. 3. Circular stapling devices are often useful. Do not assume that perfection automatically follows their use. As with sutures, staplers give results commensurate with the care with which they are used. Which to choose? The stapling device saves a little time. It may allow an anastomosis to be accomplished where suturing is difficult high in the abdomen, under the aortic arch, or high in the thorax; but if it fails, suturing is usually impossible and a higher resection is necessary. The stapling gun has an inevitable crushing effect on the tissues. If a dilated and thickened oesophagus is to be joined to the cut end of bowel, the resulting tissue bulk cannot be accommodated in the staple gun. It is safer to use a sutured anastomosis. Hand suturing is usually preferable in the neck since there may be insufficient bowel accessible below the anastomosis for insertion of the gun. 1. Have you made sure that the oesophagus and conduit, which may be stomach, jejunum or colon, can be joined without tension and are not twisted? When the oesophagus contracts the powerful longitudinal muscle causes remarkable shortening. However, longitudinal muscle is of little value in retaining sutures, which easily cut out between the muscle fibres, so the strength of the anastomosis must depend upon the submucous coat and to some extent on the mucosa. To achieve this, when dividing the oesophagus first divide the muscle coat to produce a mucosal tube 1 cm long. Divide this in the middle with one cut, leaving the mucosa/submucosa pouting and associated with a little ooze. This is the layer that must be used for the anastomosis (Fig. 8.10). 2. Make sure the hole in the conduit matches the oesophageal lumen when it is slightly stretched. Place the oesophagus and conduit together as they will lie when joined. Avoid traction stitches as they can produce tears in the oesophageal walls. The ends should lie adjacent with no tension. 3. Suture material should be absorbable, such as polyglycolic acid, monofilament polyglyconate, polydioxanone or braided polyglactin 910 or braided lactomer 9–1. Use fine material such as 3/0. 4. The argument about continuous versus interrupted stitches continues. Good surgeons obtain good results with both methods. Continuous stitches, having fewer knots that weaken the thread, can, if inserted as an unlocked spiral, appose the tissues accurately without constricting them. Interrupted sutures tied without tension have the advantage that, if one cuts out, those on either side are not necessarily prejudiced. The choice is personal, often the result of following the tenets of an admired teacher. If you use interrupted stitches, those uniting the posterior walls are tied within the lumen, those uniting the anterior walls are tied on the outer walls. 5. For interrupted sutures, place three loose full-thickness sutures at the middle of the back wall and the angles 3 o’clock and 9 o’clock (Fig. 8.11). Insert further sutures, usually between two or three, again loosely. Make sure that there are no gaps and the sutures include the mucosal layer. When the back wall is complete, tie the sutures, leaving those at the angles long. Ask the anaesthetist to pass a nasogastric tube into the conduit and secure. The anterior layer of full-thickness sutures is now placed with knots on the outside. For continuous sutures it is best to use a double-ended monofilament suture and start at the middle of the back wall. Initial sutures can be placed loose and then tightened. Working from both sides, complete the back wall and introduce the nasogastric tube as before. Complete the anterior wall and tie the suture. Buttress sutures should be avoided; they narrow the lumen and can produce ischaemia.
Oesophagus
ENDOSCOPY
Appraise
Prepare
FIBREOPTIC ENDOSCOPY
Assess
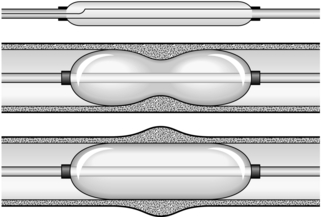
DILATATION OF STRICTURES (Fig. 8.1)
REMOVAL OF FOREIGN BODIES
Appraise
Action
INJECTION OR BANDING OF VARICES
Appraise (see Chapter 17)
INTUBATION OF TUMOURS
Appraise
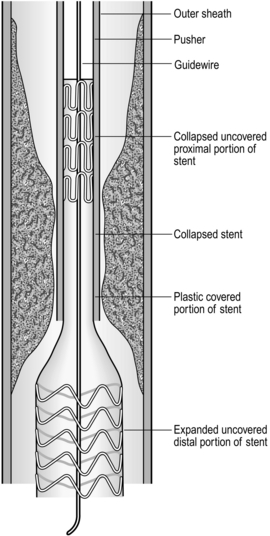
Insertion of self-expanding metal stent (Fig. 8.2)
Aftercare
OESOPHAGEAL EXPOSURE
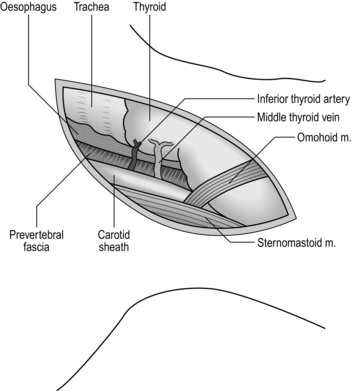
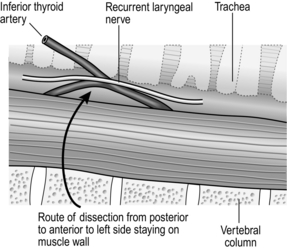
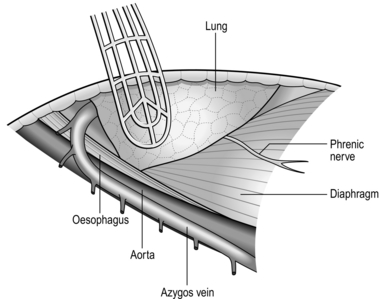
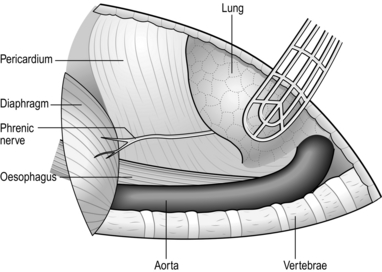
NECK (Fig. 8.3)
RIGHT POSTEROLATERAL THORACOTOMY (Fig. 8.5)
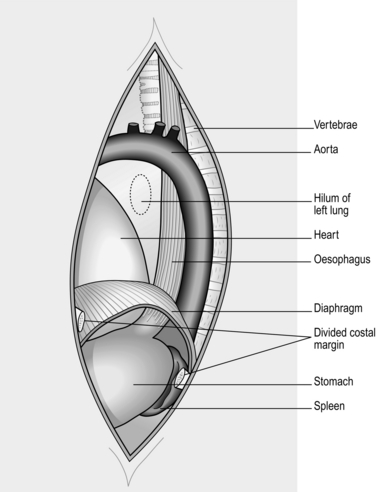
LEFT THORACOABDOMINAL APPROACH (Fig. 8.7)
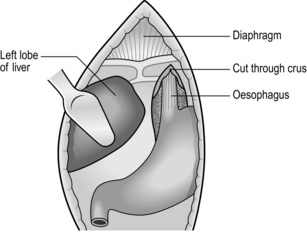
ABDOMINAL APPROACH (Fig. 8.8)
TRANSHIATAL APPROACH (Fig. 8.9)
LAPAROSCOPIC APPROACH
OPERATIVE CONSIDERATIONS
Appraise
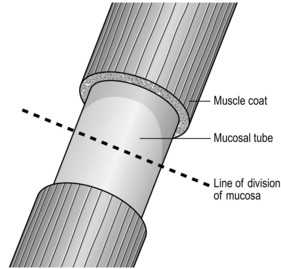
Anastomosis
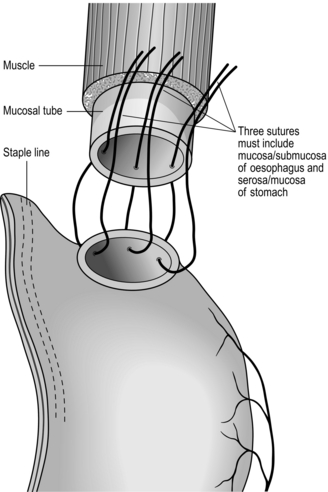
Sutured anastomosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree