Ocular drug delivery
Hala Fadda, Ashkan Khalili, Peng Tee Khaw and Steve Brocchini
Chapter contents
Anatomy and physiology of the eye
Ocular drug delivery routes and elimination pathways
Some common ocular conditions and pharmacological interventions
Topical ophthalmic preparations
Formulating ophthalmic preparations
Hydrogen ion concentration (pH)
Topical, liquid ophthalmic preparations
Topical, semisolid ophthalmic preparations
Barriers to topical ocular drug absorption
Non-corneal routes of absorption
Improving drug solubility and absorption in topical ophthalmic preparations
Sterility of ophthalmic preparations
Drug half-life in the anterior chamber
Active transporters of the cornea
Targeting the posterior segment of the eye
Periocular drug delivery routes
Problems with traditional and new ocular drug delivery systems
Patient compliance and instillation of eye drops
Key points
Introduction
Drug delivery to the eye is one of the most important areas of modern ocular therapy and presents many opportunities and challenges. The current market for ophthalmic pharmaceuticals is now worth many billions of dollars a year. The front of the eye is accessible and conditions affecting it can be treated by simple topical eye drops. The back of the eye is, however, treated as an entirely separate ocular region, and more advanced delivery systems have been designed for its treatment, including intraocular injections and implants that can provide sustained drug release over two years. A range of new therapies have and are being developed for treating ocular conditions including cells, genes and proteins, not only the traditional small molecules.
This chapter will describe the anatomy and physiology of the eye as well as the most common conditions affecting the different ocular regions. The natural anatomical ocular barriers to drug bioavailability have a great impact on ocular pharmacokinetics. Understanding ocular physiology is essential for developing drug delivery systems that are effective, safe and acceptable to patients. The design of topical ophthalmic preparations ranging from solutions to ointments and in-situ forming gels will be discussed. Although eye drops have been used since the times of Cleopatra and comprise over 90% of the ophthalmic preparations in the clinic; they have poor bioavailability and short duration of action. The reason for these shortcomings and formulation efforts to overcome them will be described.
The remainder of the chapter will focus on intraocular systems including injections and implants that deliver drugs directly to the back of the eye. This direct delivery approach has greatly improved drug bioavailability and has been approved for the delivery of several drugs. Current treatment goals are to develop systems that provide therapeutic drug levels for prolonged periods and to minimize the invasiveness of drug delivery procedures. Intraocular implants, which have been developed through sophisticated pharmaceutical, material and biomedical engineering approaches, have helped achieve these goals. Several of them are available in the clinic or are in late stage clinical trials. However, there remain unmet clinical needs, and research in this area is flourishing.
Anatomy and physiology of the eye
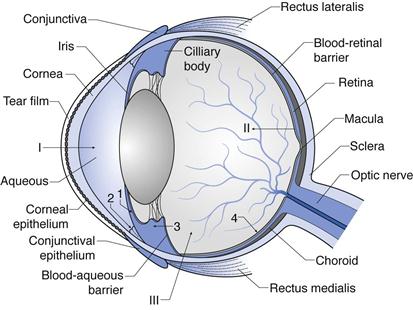
The structure of the eye is shown in Figure 41.1. This figure shows the layers and chambers of the eye and the routes and barriers to ocular drug delivery. Each of these is discussed below.
Layers of the eye
The outer layer of the eye can be considered as segments of two spheres: sclera and cornea. The sclera constitutes the back 5/6ths of the globe, and the transparent cornea provides the forward 1/6th of the globe. The sclera is a tough fibrous tissue that protects the eye from internal and external forces and maintains its shape. The front of the sclera is often referred to as the ‘white’ of the eye. The episclera is the outermost layer of the sclera and has a rich blood supply. The conjunctiva is a thin, transparent mucous membrane that covers the visible part of the sclera and extends to the inside of the eyelids. The optic nerve emerges from the sclera in the posterior part of the eye.
The cornea is the most anterior part of the eye, in front of the iris and pupil. It is densely innervated by nerves, particularly sensory nerves. While the central cornea is avascular, the region of the corneoscleral limbus is supplied by branches of the anterior ciliary arteries. The cornea refracts and transmits light to the lens and retina. It also protects the eye against infection and structural damage to the deeper parts. The cornea and sclera are connected at the limbus.
The surfaces of the cornea and conjunctiva are covered by a film of tears produced by the lacrimal gland. It lubricates the eye surface and protects it from chemicals, microbes and airborne solid particles. It comprises three layers: a mucous layer adhering to the epithelium, an aqueous layer and a superficial lipid layer. The aqueous layer constitutes electrolytes, proteins, glycoproteins, biopolymers, glucose and urea, and has a thickness of 8–12 µm. The lipid layer is composed of sterol esters, wax esters and fatty acids. The mucous layer interacts with the epithelial cells of the cornea, and so each eyelid blink allows spread of the tear film over the eye surface. A dynamic equilibrium exists in the pre-corneal tear film as it goes through a continuous cycle of production, evaporation and drainage.
The middle layer of the eyeball consists of the iris, ciliary body and choroid. The ciliary body is a ring of tissue that extends from the base of the iris to the choroid. The ciliary muscle is its most prominent structure and is in a contracted state to allow the lens to become convex. The ciliary body is also the site of production of aqueous humour. The iris is a fragile diaphragm with circular constrictor and radial dilator muscles positioned in front of the lens and ciliary body, which separates the anterior and posterior chambers. It controls the size of the pupil and thus the amount of light reaching the retina. The colour of the iris is determined by the amount of melanin expressed in it. The choroid is the vascular layer of the eye lying between the retina and sclera. It provides oxygen and nutrients to the outer layers of the retina.
The inner layer of the eye is the retina, which is a complex network of neurons that process light. The layer of the retina surrounding the vitreous cavity is the neural retina, the outer retinal wall surrounded by the choroid and sclera is the retinal pigment epithelium (RPE). The neural cells of the retina are arranged in several parallel layers and the major classes present are photoreceptors (the rods and cones that are responsible for the conversion of light into an electrical signal through the presence of pigments), bipolar cells, horizontal cells, amacrine cells, ganglion cells (which capture and process light signals) and the Müllerian glia (which form the organizational backbone of the neural retina). The RPE constitutes about 3.5 million epithelial cells arranged in a hexagonal pattern. Their important functions include the maintenance of photoreceptor function, storage and metabolism of vitamin A, production of growth factors required by nearby tissue, and wound healing after injury or surgery.
Chambers of the eye
The eye contains three main chambers: anterior chamber, posterior chamber and vitreous cavity. Aqueous humour fills the anterior and posterior chambers. It is a clear, colourless, watery fluid that comprises a vast array of electrolytes, organic solutes, growth factors and other proteins that nourish the non-vascularized tissue of the anterior chamber; particularly trabecular meshwork, lens and corneal endothelium. It is produced by the ciliary body epithelium and flows into the anterior chamber. Aqueous humour leaves the anterior chamber through the trabecular meshwork into Schlemm’s canal and aqueous veins (conventional pathway) or through the ciliary muscle and other downstream tissues (unconventional pathway). If the exit of aqueous humour from the eye is blocked, the amount of fluid within the eye increases, leading to an increase in pressure which may lead to glaucoma and cause damage to the optic nerve. The trabecular meshwork is made up of an extracellular matrix forming a porous like structure through which aqueous humour flows into the canal of Schlemm. Schlemm’s canal connects with the venous system through a network of 25 to 35 collector channels.
The vitreous cavity comprises 80% of the volume of the eye. It weighs approximately 3.9 g and contains vitreous humour. This is a hydrogel containing approximately 98% water. The other 2% of vitreous components are predominantly collagen fibrils and hyaluronic acid. Proteins, inorganic salts, glucose and ascorbic acid are also present. Vitreous humour has a pH of approximately 7.5 and a viscosity 2–4 times that of water. The presence of sodium hyaluronate is primarily responsible for the viscosity of the vitreous humour. Its viscous properties allow it to return to its normal shape when compressed. The vitreous body is surrounded by a thin membrane known as the hyaloid membrane and no blood vessels penetrate it; its nutrition is therefore carried by vessels of the retina and the ciliary body.
Ocular drug delivery routes and elimination pathways
Routes and barriers to ocular drug delivery can be summarized here with reference to Fig. 41.1 (see I, II and III).
Ocular drug elimination (Fig. 41.1) is via:
1. drug elimination from the aqueous humour into the systemic uveoscleral circulation
2. aqueous humour outflow through the trabecular meshwork and Schlemm’s canal.
3. Drug elimination from the vitreous humor via diffusion into the anterior chamber.
4. Drug elimination via posterior route across blood retinal barrier.
Some common ocular conditions and pharmacological interventions
Ocular drug delivery is undertaken for treatment of local disease at different sites in the eye, thus a brief introduction to common eye conditions is appropriate.
Dry eye syndrome.
Dry eye is a common disease which occurs when either the tear volume is inadequate or of poor quality (poor functional tear). This often results in unstable tears and consequently ocular surface disease. Dry eye is not curable and management is to control the symptoms and protect the ocular surface from being damaged. The initial treatments include use of tear substitutes and mucolytic eye drops. In advanced cases, the use of anti-inflammatory eye drops, surgical intervention to reduce punctual drainage and contact lenses have been shown to be beneficial.
Cataract.
Cataract is the opacity of lens, which often results from denaturation of the lens protein. Cataracts, which are usually age-related, are the most common cause of treatable blindness worldwide. Surgery is the only treatment and is very effective. It involves replacement of the clouded lens with a synthetically produced intraocular lens.
Glaucoma.
The glaucomas are a group of diseases in which there is a specific type of damage to the optic nerve (optic disc cupping), resulting in a characteristic pattern of visual field loss; first peripheral and then central vision loss. Glaucoma, a life-long condition, is the leading cause of irreversible blindness worldwide and is the second most common cause of blindness, after cataract. The most important and only modifiable risk factor in this group of diseases is raised intraocular pressure (IOP). It has been shown that reduction in intraocular pressure, medically (eye drops) or surgically, can halt or decrease the progression of the visual field loss.
Age-related macular degeneration (AMD).
AMD is a degenerative disorder that affects the macula, the most sensitive part of the retina, and consequently results in the loss of central vision. AMD is the leading cause of irreversible visual loss in industrialized countries and often occurs in the population over the age of 50 years. There are two forms of AMD: wet and dry. Wet AMD arises when abnormal new blood vessels grow underneath the macula and leak, thus raising the macula off the back of the eye and causing loss of central vision, often very quickly. With the more common dry AMD, the light-sensitive cells in the macula degenerate, with a gradual blurring of central vision. Recent anti-vascular endothelial growth factor (anti-VEGF) treatments including pegaptanib (Macugen) and ranibizumab (Lucentis) have shown beneficial effects in the majority of patients with wet AMD. This treatment, however, requires multiple intraocular injections with an average of 8–10 injections per year (this is discussed in detail in a later section).
Endophthalmitis.
Endophthalmitis is the inflammation of the internal layers of the eye. Infectious endophthalmitis most frequently occurs following ocular surgery and penetrating trauma, particularly with retained foreign body. Special care must be taken when injecting medicines into the back of the eye to ensure complete sterility is maintained. The most commonly cultured bacteria in post-operative endophthalmitis are gram positive (90%), including Staphylococcus epidermidis, Staphylococcus aureus and Streptococcus species. The most common bacteria found following trauma are staphylococcus and bacillus species. Non-infectious endophthalmitis may occur due to many causes including impurities in intraocular injections (e.g. endotoxin, silicone oil precipitates). The main treatments are antibiotics (peri-orbital, intra-ocular and parenteral).
Topical ophthalmic preparations
Topical ophthalmic preparations include: solutions, suspensions, ointments/gels and the newer dispersion systems. These have been traditionally used for treating pathological conditions of the anterior segment, such as infection, inflammation, allergy, dry eye, glaucoma, and corneal ulceration, as well as for instilling anaesthetic and diagnostic agents.
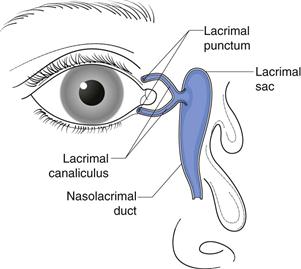
Designing ocular drug delivery formulations requires an understanding of what can be tolerated by the eye. Physiological and biochemical mechanisms exist to protect the eye from harmful stimuli. While these mechanisms are protective, they do sometimes present a barrier to drug absorption. The lacrimal system of the eye is extremely dynamic. The tear volume in the normal eye is 5–9 µL. Basal tears are continuously secreted by the lacrimal glands at an average rate of 1.2 µL/minute, thus giving a tear turnover rate of about 17%/minute. Reflex tears are triggered by irritants and can vary from 3 to 400 µL/minute, the intention being to rapidly eliminate the stimulus. Another protective mechanism is the eyelid movements associated with blinking. Blinking moves tear fluids and foreign matter to the nasal corner of the lid surface, where the liquid exits via the puncta and is then drained away by the nasolacrimal ducts into the inferior nasal passage (Fig. 41.2). Tears contain lysozymes and immunoglobulins which impart an anti-infectious activity. The combined mechanisms of lacrimal drainage and blinking means that administered eye drops are rapidly cleared from the conjunctival sac with residence time ranging from 4 to 23 minutes. Moreover, the rate of drainage from the eye has a positive, linear correlation with instilled volume. It has been found that the palpebral fissure in the open eye is capable of accommodating only 20 to 30 µL of added fluid temporarily without spilling.
Ideally, administered eye drops should be spaced by several minutes to minimize washout. Punctal occlusion maximizes local absorption and minimizes unnecessary systemic exposure by up to 70%. To reduce the elimination rate of administered eye drops, it is important that the topical preparations do not cause irritation. This can be achieved by designing their properties to be as close as possible to the lacrimal fluids covering the surface of the eye.
Formulating ophthalmic preparations
Osmolality
The concentration of salts in lacrimal fluids determines its osmolality. Predominant inorganic ions in tears are sodium, potassium, calcium, chloride and bicarbonate. These have an important function in controlling the osmotic pressure of the intercellular and extracellular fluids of the epithelial spaces of the cornea and conjunctiva. Osmolality in healthy, non-dry eyes has an average value of 302 mmol/kg during the daytime. Patients with dry eye syndrome have been found to present with tear film hyperosmolality which contributes to the symptoms of the disease.
When the eye is exposed to a hypotonic ophthalmic solution, the corneal epithelium becomes more permeable and water flows into the cornea causing oedema. Hypertonic solutions have a dehydrating effect on the corneal epithelium. Hypotonic and hypertonic solutions are irritating to the eye and therefore induce an increased production rate of tears. The rate of tear production increases to several hundred microlitres per minute through reflex tear secretion and reflex blinks. This increased tear turnover rate reduces the retention half-life of a solution that has been applied to the eye.
Normal tear osmotic pressure is equivalent to 0.9% to 1.0% sodium chloride solution. Solutions of osmotic pressure equivalent to 0.6 to 1.3% sodium chloride appear to be well tolerated by the eye. Ophthalmic solutions can be made isotonic by the use of tonicity agents such as sodium chloride, potassium chloride, buffering salts, dextrose, mannitol and glycerol, as long as they are compatible with the other ingredients in the formulation.
Hydrogen ion concentration (pH)
The pH of tears is close to neutral and is controlled by various substances dissolved in the aqueous layer of tears: carbon dioxide, bicarbonate and proteins such as the basic lysozyme and an acidic tear prealbumin. The fatty acids produced by the Meibomian glands also mix with the aqueous phase of tears. The pH of tears is subject to diurnal variation and increases slowly from 6.9 to 7.5 during the waking hours of the day due to carbon dioxide evaporation. The buffer capacity of tear fluids is low but significant; it is predominantly controlled by the balance of bicarbonate and carbon dioxide, as well as proteins. Acidic or basic solutions instilled into the eye cannot be neutralized by the tears that are present and therefore reflex tears are generated to dilute the administered drop and eliminate it. The recovery to the original pH of the tear film can vary from a few minutes up to 20 minutes. The duration of recovery is influenced by the pH, volume, and buffer capacity of the administered solution, as well as the age of the patient. Strongly acidic or basic solutions should not be administered to the eye as they can cause damage to the ocular tissue. The eye can generally tolerate topical ophthalmic preparations at a pH within the range of 3.5 to 9. However, it is preferable to formulate as close to physiological tear pH as possible to reduce discomfort and the associated increased lacrimation.
pH is important in drug ionization and product stability. Pilocarpine is a natural alkaloid used in the treatment of glaucoma. It undergoes pH-dependent hydrolytic degradation and one of the ways to maintain stability of pilocarpine aqueous solution is to maintain the pH at 4–5 through the use of a weak acidic buffer. Since the pH deviates from the physiological pH of the lacrimal fluids, the constituting buffer must be weak to allow the lacrimal fluids to be restored to their normal pH within a short period of time following instillation. Drug ionization is also important in determining drug solubility and permeability across the corneal epithelium. The extent of ionization can be manipulated through control of the pH of ophthalmic preparations.
Commonly used buffers in ophthalmic solutions include borate and phosphate buffers. To prepare solutions of lower pH range acetic acid/sodium acetate and citric acid/sodium citrate buffers are used. It is important that strong buffers are not used and to use a low concentrations of weak buffers.
Surface tension
The surface tension of tear fluid at physiological temperature in a healthy eye is 43.6 to 46.6 mN m-1. Administration of solutions that have a surface tension much lower than that of the lacrimal fluid destabilizes the tear film and disperses the lipid layer into droplets that are solubilized by the drug or surfactants in the formulation. The oily film reduces the rate of evaporation of the underlying aqueous layer and therefore once it is lost, dry spots are formed which are painful and irritant. Surfactants are implicated in this.
Surfactants are typically included in ophthalmic preparations to solubilize or disperse drugs. Irritation power of surfactants decreases in the following order: cationic > anionic > zwitterionic > non-ionic. Non-ionic surfactants are therefore the most commonly used, examples include; polysorbate 20, polyoxyl 40 stearate, polyoxypropylene-polyoxyethylenediol. Despite being the least irritant, non-ionic surfactants have been shown to remove the mucus layer and disrupt the tight junctional complexes of the cornea; therefore increasing drug permeability. Surfactants may also interact with polymeric substances in the preparation and reduce the efficacy of preservatives. The concentration of surfactant is important not only in terms of drug solubility, safety and patient tolerance, but also because high concentrations can lead to foaming upon product manufacture or shaking.
Viscosity
Viscosity enhancing polymers are used in ophthalmic solutions to prolong drug retention in the precorneal tear film and thus enhance drug absorption. Mechanisms proposed are not just reduced drainage rate; the thickness of the precorneal tear film is also increased due to the ability of viscosity-enhancing polymers to drag water and stabilize the aqueous layer as they spread over the corneal surface on blinking. This increased volume acts as a reservoir for the drug so that it is re-spread in the tear film over the cornea with each blink. Water soluble polymers that have been used to increase solution viscosity include poly (vinyl alcohol), poly (vinylpyrrolidone), various cellulose derivatives, particularly; methylcellulose, hydroxypropyl methylcellulose and carboxymethyl cellulose (at concentrations of 0.2–2.5%) and poly(ethylene glycol)s (at concentrations of 0.2–1%).
Tears are non-Newtonian fluids whose coefficient of viscosity is shear dependent (shear-thinning). This is commonly seen with linear, multiple-charged polymers such as sodium hyaluronate and Carbopol. Zero shear viscosity values of 4.4 to 8.3 mPa s have been reported for normal tears. The force required by the eyelids to blink is 0.2 N and for a forceful blink it is 0.8 N. The pain threshold is 0.9 N and therefore if a higher force than this is required for blinking, then it would be painful for the patient. This limits the acceptable viscosity of administered ocular solutions since the force needed to move the instilled solution at the shear rates equivalent to those generated by blinking should be lower than 0.9 N. Furthermore, very viscous solutions can cause blurring of vision and may block the puncti and canaliculi. Nevertheless, solutions containing viscoelastic material can be used at higher viscosities. Since the viscosity of viscoelastic polymers is shear dependent; the viscosity of these polymer solutions can change in the eye due to blinking.