If there will be no exposure to a substance, no matter how hazardous it may be, there is no risk of harm. Substances which pose only a small hazard but to which there is frequent or excessive exposure may pose as much risk as substances which have a high degree of hazard but to which only limited exposure occurs.
The terms hazard and risk are often used interchangeably, however these are two very distinct terms. Basically, a hazard is anything that can cause harm or adverse effects. A hazard poses no risk if there is no exposure to that hazard.
Health risk assessment is the process of examining and evaluating the risk to the health and safety of workers while at work arising from the circumstances of the occurrence of a hazard at the workplace [1]. A risk assessment is a careful examination of what, at the workplace, could cause harm to people, so that the employer can decide whether he has taken enough precautions or should do more to prevent harm. Workers and others have a right to be protected from harm caused by a failure to take reasonable control measures. Accidents and ill health can ruin lives and affect the business too if for example output is lost, machinery is damaged, insurance costs increase or the employer even have to go to court.
As a rule risk levels are treated for setting priorities: to decide about the risks that have to be mitigated in the first place.
If a manufacturer will be reproached or even sued by any competent body, it will be based on a societal decision about the level of risks that is not (any more) accepted by a group or nation: ‘society’.
26.2.1 Categorisation
The risk of each work situation with exposure to substances is unique: it depends on the hazards of the substances, on the exposure of the worker to these substances and on the physical condition and constitution of the worker. However, it is practically impossible to investigate the risk for any individual in any situation. Therefore categorisation is applied to exposure as well as to hazards of substances and even individuals. With categorised hazards and exposures, also the risks (= hazard × exposure) are categorised. Because several uncertainties will affect the ‘accounting’ of the risk, the risk is not an exact characteristic, level or value, although qualifications as: ‘high’, ‘medium’ and ‘low’ can be given.
26.2.2 Containment
Containment is an engineering control measure, the second risk mitigation principle (see Sect. 26.7.1). It may be defined as a process or device to contain product, dust or contaminants in one zone, preventing it from escaping to another zone [2] or, to put it differently, the prevention of the escape of a substance.
26.3 Hazard of Pharmaceutical Substances
26.3.1 Definition
This section discusses the hazard that may be created by substances used to produce medicines, with emphasis on workers in health care. These workers are mainly pharmacists, operators, nurses and doctors.
Every substance can present a hazard in general. In principle, being ‘hazardous’ is a consequence of one or more intrinsic hazard properties of a substance. In accordance with the Classification, Labelling and Packaging (CLP) Regulation [3], see also Sect. 26.6.3, hazardous substance are those substances that fulfil the criteria of at least one hazard class. The hazard classes comprise physical hazards, health hazards or environmental hazards (see Sect. 26.3.2).
However, the use of the term ‘hazardous substances’ with regard to pharmacy preparation and reconstitution, is often restricted to carcinogenic, mutagenic and reprotoxic substances. Radiopharmaceuticals and gene therapeutics may be counted under this term as well. The National Institute of Occupational Safety and Health of the US (NIOSH) however also counts substances with a high chronic toxic potential to ‘hazardous substances’, see further Sect. 26.3.3.
So the term hazardous substances may have different notions. In this chapter the CLP definition is followed meaning that all substances are considered potentially hazardous. Carcinogenic, reprotoxic and mutagenic substances are either noted as such or as a group as CMR. Occupational safety and health care investigates all processes and all substances, to prevent health damage of workers.
26.3.2 Hazard Types
Hazards of substances can be acute, for example if a strong acid is spilled on the skin, or chronic, if long term exposure results in health damage, such as sensitisation or cancer.
The Globally Harmonised classification and labelling System (GHS, see Sect. 26.6.3), discerns three major hazard groups:
Physical hazards: hazards that are directly safety-threatening, for example, substances and mixtures that are (in)flammable or explosive (such as ether and ethanol).
Health hazards: Firstly direct hazards (acute toxicity), such as from substances that are irritating (trichloroacetic acid, sodium hydroxide), intoxicating (ether) or suffocating. Secondly hazards that arise in the longer term (chronic toxicity), such as damage to the respiratory system, the nervous system or reproductive organs in the long term. Also skin and airway allergies and cancer belong to this type of hazards.
Within chronic health hazards a further distinction is made into:
Carcinogenic: may induce cancer or increase its incidence
Mutagenic: may induce heritable genetic defects or increase their incidence
Reprotoxic: may produce or increase the incidence of non-heritable adverse effects in the offspring and/or causes either a decrease in fertility or problems with foetal development
Respiratory sensitising/inhalant allergenic: causes allergy via inhalation
Environmental hazards: for example substances and mixtures that are directly or in the long term a hazard to aquatic life or that are poorly degradable. This group also include hazards to the ozone layer. Environmental hazards are further dealt with in Chap. 38.
26.3.2.1 Hazard and Precautionary Statements in GHS
The GHS defines Hazard and Precautionary statements (in brief H- and P-statements), which represent standard phrases used to respectively describe the hazards of hazardous substances and mixtures and the recommended measures to be taken when using/disposing of hazardous substances and mixtures. The GHS couples any H-statement to a P-statement. These statements will assist in ensuring that all users of the products, worldwide, will know and understand proper precautionary measures when interacting with the chemicals.
H- and P-statements are one of the key elements for the labelling of containers under the GHS. Some H-statements are depicted as EUH-statements (these are a classification from previous legislation). These apply, legally, only to European countries and are based on CLP regulations, see further Sect. 26.6.3.
According to the Regulation (EC) No 1272/2008 [3], the substance morphine hydrochloride is for example provided with the following hazard en precautionary statements [6]:
H302 | Harmful if swallowed |
H336 | May cause drowsiness or dizziness |
P261 | Avoid breathing dust/fume/gas/mist/vapours/spray |
P264 | Wash hands and other exposed areas thoroughly after handling |
P301 + P312 | IF SWALLOWED: call a POISON CENTER or doctor/physician if you feel unwell |
P304 + P340 | IF INHALED: remove victim to fresh air and keep at rest in a position comfortable for breathing |
P312 | Call a POISON CENTER/doctor/physician if you feel unwell |
P330 | Rinse mouth |
P403 + P233 | Store in a well-ventilated place. Keep container tightly closed |
P501 | Dispose of contents/container to: Hazardous waste. Comply with applicable regulations |
In addition, the signal word ‘Warning’ and a pictogram with a white background, a red frame and an exclamation mark identify morphine hydrochloride.
Each packaging must be labelled with the relevant hazard pictograms, signal word and H- and P-statements. More detailed hazard and precautionary information can be found in the material safety data sheets (MSDS) of the substance.
26.3.3 Carcinogenic, Mutagenic and Reprotoxic (CMR) and Sensitisation Hazards
The carcinogenic, mutagenic and reprotoxic hazards are together called CMR hazards. Substances that are carcinogenic, mutagenic or reprotoxic (CMR substances) are of very high concern due to the long term and serious effects that they may exert on human health. Within the regulation on Registration, Evaluation, Authorisation and Restriction of Chemicals, Regulation (REACH) (see Sect. 26.6.2) CMR substances are classified and listed in Annex VI of the EU GHS Regulation [4]). Substances appear on this list if they are registered under REACH or notified under the CLP regulation (see Sect. 26.6.3) or both. The classification in Annex VI is legally binding for Europe. National authorities may have extended this list with substances they consider being relevant [7].
The National Institute for Occupational Safety and Health (NIOSH) of the United States describes in the section ‘Determining whether a drug is hazardous’ how human and animal data on carcinogenicity, reprotoxicity and genotoxicity are interpreted for their list of hazardous substances [8].
26.3.3.1 Classification of Carcinogens
According to CLP, CMR substances are classified into three categories based on the strength of evidence showing that they present one of the CMR types of hazards to human health. Apart from the European CMR classification, there are other classifications, in particular the classification system established by the International Agency for Research on Cancer (IARC). This classification includes agents, groups of agents, mixtures and carcinogenic exposure circumstances. It includes four groups of classifications, based on different levels of scientific evidence of carcinogenicity for humans.
European CMR classification [5]:
Category 1A: Classification is largely based on human evidence.
Category 1B: Classification is largely based on animal evidence.
Category 2: Classification is based on the evidence obtained from human and/or animal studies, but which is not sufficiently convincing to place the substance in Category 1A or 1B.
Carcinogenic classification from IARC [9]:
Group 1: “Carcinogenic to humans” There is enough evidence to conclude that it can cause cancer in humans.
Group 2A: “Probably carcinogenic to humans” There is strong evidence that it can cause cancer in humans, but at present it is not conclusive.
Group 2B: “Possibly carcinogenic to humans” There is some evidence that it can cause cancer in humans but at present it is far from conclusive.
Group 3: “Unclassifiable as to carcinogenicity in humans” There is no evidence at present that it causes cancer in humans.
Group 4: “Probably not carcinogenic to humans” There is strong evidence that it does not cause cancer in humans.
The corresponding H-statements (see Sect. 26.3.2) for CMR substances, according to the European CMR classification, are depicted in Table 26.1.
Table 26.1
Correspondence between H-statements and European CMR classification
Category 1A or 1B | Category 2 | Effects on or via lactation | |
|---|---|---|---|
Carcinogens | H350: May cause cancer | H351: Suspected of causing cancer | |
Mutagens | H340: May cause genetic defects | H341: Suspected of causing genetic defects | |
Reprotoxics | H360: May damage fertility or the unborn child | H361: Suspected of damaging fertility or unborn child | H362: May cause harm or to the breast-fed children |
26.3.3.2 Carcinogenicity and (Non-)Genotoxicity
Apart from differentiation on the basis of the level of evidence, as done by categories 1A, 1B and 2, another distinction within the category carcinogenic substances can be made: whether they are genotoxic or non-genotoxic.
Genotoxic carcinogens interact directly with DNA, causing damage to DNA which may lead or contribute to cancer development. Non-genotoxic carcinogenic substances have no direct interaction with DNA but are capable of influencing by one of many secondary mechanisms the proliferation process of the tumour, thereby leading or contribute to cancer. The action of non-genotoxic substances is subjected to a threshold; below a certain concentration there will be no effect (No Observed Effect Level, NOEL). By definition for genotoxic carcinogens no safe threshold without a theoretical cancer risk can be derived.
This difference between genotoxic and non-genotoxic carcinogens brings about a principally different approach to protection. If exposure to non-genotoxic substances does not exceed the threshold value, humans are considered to be safe from getting cancer from those substances, just as is the approach regarding other toxic substances having a threshold value. Exposure to even one molecule of a genotoxic substance however may lead to cancer, although the chance that it occurs is limited. A decision about that limited level of risk is made by society. This leads to an acceptable level of exposure, rather than a threshold, to keep a distinction between both approaches. With a genotoxic carcinogen, linear extrapolation is used for estimating the risks associated with a given level of exposure [10].
For pharmacy practice this distinction between genotoxic and non-genotoxic carcinogenic substances is at the moment of little practical value, because only very few carcinogenic active substances have been defined as non-genotoxic.
Among the most widespread non-genotoxic carcinogens are a group of compounds collectively referred to as peroxisome proliferators. Peroxisome proliferators are a diverse class of chemicals, including the lipid and cholesterol lowering fibrate drugs (clofibrate, fenofibrate, and gemfibrozil), plasticisers (phthalate esters), solvents (e.g., trichloroethylene), and naturally occurring chemicals (e.g., phenyl acetate) or hormones (e.g., dehydroepiandrosterone sulfate) [11].
Chloroform is another example of a non-genotoxic carcinogen. In most European countries chloroform has a 8 h limit value (see Sect. 26.7.2) of 10 mg/m3 [12].
As a summary Table 26.2 lists the characteristics of genotoxic and non-genotoxic carcinogenic substances.
Table 26.2
Characteristics genotoxic and non-genotoxic substances (From Klaunig et al. [13] with permission)
Genotoxic carcinogens | Non-genotoxic carcinogens |
|---|---|
Mutagenic | Non-mutagenic |
Direct DNA reactivity | Non-directly DNA reactive |
Tumorigenicity is dose response | |
Threshold?? | Exhibits threshold |
Can be complete carcinogens | |
Irreversible | Reversible |
Usually not strain or species specific | Usually exhibits strain, species and tissue specificity |
Functions at initiation and progression stages of cancer process | Functions at the tumour promotion stage of the cancer process |
Examples: nitrosamines, polycyclic aromatic hydrocarbons, aromatic amines | Examples: chlorinated substances, organochlorine pesticides, hormones, barbiturates |
It is to be noted that the decision on a threshold and a non-threshold mode of action may not always be easy to make, especially when, although a biological threshold may be postulated, the data do not allow identification of it. If not clear, the assumption of a non-threshold mode of action would be the prudent choice.
Establishing exposure limits with genotoxic and non-genotoxic substances, is dealt with in Sect. 26.7.2.
26.3.3.3 Reprotoxicity
Next to the classification based on the evidence, also for reprotoxic substances two types of toxicity can be discerned:
1.
Mutagenic: causes damage to genetic material in sperm and ova;
2.
Teratogenic: causes damage to the unborn child.
The concepts of genotoxicity and mutagenicity are sometimes used interchangeably, but do not have the same content. All mutagens are genotoxic, however not all genotoxic substances are mutagenic. Genotoxic substances can cause damage to DNA in different ways, but only when there has been damage to DNA in sperm and ova, are they mutagenic. Mutagenicity is thus a form of genotoxicity (Fig. 26.1).
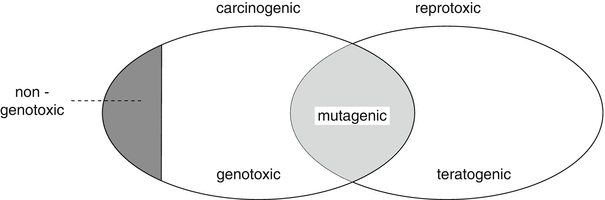

Fig. 26.1
The relation between mutagenicity and genotoxicity
26.3.3.4 Classification of Monoclonal Antibodies (mAbs)
A decade ago all antineoplastic agents (ATC class L01) were considered to be carcinogenic. That is no longer the case, especially if monoclonal antibodies (mAbs) are considered.
NIOSH re-evaluated the inclusion of monoclonal antibodies as hazardous substances because of their specific targeted mechanisms of action and their high molecular weight that prevent skin penetration and accidental inhalation. The 2014 NIOSH list of hazardous substances [8] contains now only mAbs conjugated with antineoplastic active substances.
A German working group evaluated whether mAbs are to be classified as carcinogens, mutagens or reprotoxic at dermal, oral or inhalative exposure. Sensitising properties were evaluated as well. They assigned H-statements to mAbs based on a systematic literature review, European public assessment reports (EPARs) and data provided by national and international occupational safety and health organisations. Expert opinions were also obtained. Recommendations for the protection of workers handling mAbs were agreed with experts in the fields of chemistry, pharmacy and occupational safety as well as representatives of the pharmaceutical industry. They happened to classify some mAbs as reprotoxic however not as carcinogenic [14, 15].
Swiss hospital pharmacists [16] do not consider monoclonal antibodies as hazardous for healthcare practitioners and only gloves are recommended for their manipulating at wards.
26.3.3.5 Respiratory Sensitisation
Exposure to inhalant allergens can lead to respiratory allergies. This generally begins with sensitisation (= to become sensitive to the relevant substance). Eventually, however, an allergy develops that is harmful to health, even when exposure is minimal. The clinical description of this process is to be found in [10]. For the endpoints sensitisation and irritation it is supposed that a substance exerts its effect by a threshold mode of action, but the available data do not allow a reliable identifcation of the threshold [10]. A situation similar to that of genotoxic carcinogens occurs. This will lead to a similar risk-based approach to setting exposure levels (see Sect. 26.7.2).
26.3.4 Information Sources on Hazards
Information on hazards of substances may originate from several sources. The first recommended source is the Safety Data Sheet (SDS) of substances and mixtures, under the REACH regulation. The second source may be human toxicological and pharmacologic data.
26.3.4.1 Safety Data Sheets
The SDS contains information on the identity of the substance or the mixture, potential health effects, toxicological properties, physical hazards, safe use, handling and storage, emergency procedures, and disposal requirements specific to the chemical (see Fig. 26.2). Intermediate products are to be considered as mixtures of substances.
For some products, such as licensed medicines, safety data sheets do not have to be provided [18] but sometimes suppliers provide them anyhow. So if pharmacists or nurses have to manipulate oral dosage forms they usually cannot refer to a SDS but sometimes they can. The Summary of Product Characteristics (SmPCs) usually doesn’t provide information for these situations. In the United States so-called manufacturers’ safe handling guidance (MSHG) are available for some medicines, including antineoplastics [19] and in section 16 of the Label information leaflet a safe-handling warning may be given.
Reliability of SDSs
The compilation of a good SDS requires extensive knowledge in different fields, as the SDS itself covers a wide range of aspects concerning the substance or mixture properties, occupational health and safety, transport safety and environmental protection. REACH indicates that the SDS should be compiled by a competent person, but no specific definition of competent in this context is given in the Regulation [20].
The SDS is commonly first compiled by the manufacturer but the requirements of REACH in relation to the provision of SDSs apply at each stage of the supply chain. Any supplier of a substance or mixture must provide a SDS for it. Each supplier remains responsible for the accuracy of the information in the SDS they provide even though they may not have prepared the safety data sheet.
Some of the SDSs found on the Internet may be of questionable quality or may not be the most current version. The manufacturer is often the best source of the current and accurate SDS. Employers may rely on the information received from their suppliers. If receiving one that is obviously inadequate, an appropriately completed one should be requested.
A number of studies and investigations have raised concern that some SDSs may be incomplete or contain erroneous or out-of-date information. The U.S. Occupational Safety and Health Administration (OSHA) has confirmed there are inaccurate SDSs in circulation, but a comprehensive study on this topic that provides more than anecdotal evidence about a limited number of SDSs has not been performed [21].
The authors consider the SDSs for the Ph. Eur. reference substances [22] as a reliable source of SDSs for pharmaceutical substances.
26.3.4.2 Human Toxicological and Pharmacologic Data
For occupational safety and health information for the preparation of pharmaceutical products a second valuable source about hazards is the information on pharmacologic and toxicological effects on humans of active substances and excipients. This information can be used with some restrictions:
For occupational safety and health, the inhalation and dermal routes are most interesting; most therapeutic information is however about oral and parenteral administration.
Any adverse effect is accepted (or not) by a patient in relation to his illness; for workers the same effect usually weighs more heavily because it is related to a long-lasting work situation.
26.3.5 Categorisation of Hazards of Substances
Categorisation of hazards of substances (also called ‘control banding’) instead of considering every substance separately serves practical work procedures. Two approaches are met:
Categorisation of all substances based on grouping H-statements and making use of human toxicologic and pharmacologic data
Categorisation of the most toxic substances by a procedure with public consultation (NIOSH)
26.3.5.1 Categorisation Based on the GHS and Human Toxicology and Pharmacology
Based on the ideas of Naumann [23]: categories of hazards of pharmaceutical substances have been distinguished by 3 (groups of) investigators: Ader et al. [24, 25], Tielemans et al. [26] and Vincent et al. [27]. They all use a comparable approach: 4 or 5 categories of increasing hazards with category 1 representing the lowest grade with the (relatively) lesser hazards and the highest category (which is 4 or 5 depending on the model) containing the most serious hazards. Only Tielemans [26] and Vincent [27] make use of the GHS hazard statements. All of them make use of human toxicological and pharmacologic information.
H-statements (in fact the original R-phrases) have been divided over the different categories guided by the COSHH (Control of Substances Hazardous to Health) essentials scheme [28], mainly led by the height of exposure giving toxic effects, the presence of a threshold value, the severity of the effect and reversibility of the effect.
Table 26.3 reflects the general approach that all three sources follow. About category 1 Tielemans [26] says: If there is no exposure limit value, no H-statements to attach, no dosage details are known and it is plausible that the substance causes no harm; the substance is classified into hazard class 1. Whereas Ader [24] considers category 4 as the ‘default category’, to be understood as: if there are any doubts about harmlessness, consider it to belong to category 4.
Harm characterisation | Category 1 | Category 2 | Category 3 | Category 4 | Category 5 |
|---|---|---|---|---|---|
Very low | Low | Intermediate | High | Extremely high | |
Reversible | Reversible | Irreversible effects | Irreversible effects | ||
Low acute or chronic toxicity | Systemic toxicity | Carcinogenicity, mutagenicity, reprotoxicity | Genotoxic carcinogenicity, mutagenicity, reprotoxicity | ||
Pharmacological effect, potency | Very low > 100 mg/day | Low (effect at 10–100 mg/day) | Moderate (toxicity at 0.1–10 mg/day) | Significant potency (effect ~ 0.01–1 mg/kg or 0.1–1 mg/day clinical dose) | Highly potent pharmacological effect (~10 micrograms/kg or < 0.1 mg/day) |
Example of adverse effect | Irritant to the skin or eyes | Weak (skin or respiratory) sensitisers | Sensitisers | Severe sensitisers | |
Approximate OEL [24] | >0.5 mg/m3 | 10 micrograms/m3 to 0.5 mg/m3 | 30 ng/m3 to 10 micrograms/m3 | <30 ng/m3 | |
Approximate OEL [26] | >5 mg/m3 | 1–5 mg/m3 | 10–1,000 micrograms/m3 | 1–10 micrograms/m3 | <1 micrograms/m3 |
The relation between hazard statements and classes of the system of Tielemans [26] are given in Table 26.4, with the notion that the translation of the R-statements into the H-statements, is the authors’ view.
Criterium | Category (hazard class) | ||||
|---|---|---|---|---|---|
1 | 2 | 3 | 4 | 5 | |
Therapeutic dose (mg/day) | >100 | 10–100 | 0.1–10 | 0.1 | <0.1 |
Occupational Exposure Limit value (micrograms/m3) | >5,000 | 1,000–5,000 | 10–1,000 | 1–10 | ≤1 |
Acute toxicity | H304 | H302 | H301 | H300 | H370 (R39/26, R39/26/27, R39/26/27/28, R39/26/28, R39/27, R39/27/28, R39/28) |
H336 | H312 | H311 | H310 | ||
H332 | H331 | H330 | |||
H371 | H370 (R39/23, R39/23/24, R39/23/24/25, R39/23/25, R39/24, R39/24/25, R39/25) | ||||
Irritation (skin, respiratory tract and/or eyes) | H315 | H314 | |||
H319 | H318 | ||||
EUH066 | H335 | ||||
Sensibilisation (skin and or respiratory tract) | H317 | ||||
H334 | |||||
Chronic toxicity | H373 | H372 | |||
Mutagenicity | H340 | ||||
H341 | |||||
Carcinogenicity | H351 | H350 | |||
H350i | |||||
Reproduction toxicity | H360F | ||||
H360D | |||||
H361f | |||||
H361d | |||||
Flammability | H224 (flash point < 23 °C and boiling point ≤ 35 °C) | H224 (boiling point ≤ 35 °C) | H220 | ||
H225 (flash point < 23 °C and boiling point > 35 °C) | H225 (boiling point > 35 °C) | H221 | |||
H226 (flash point ≥ 23 °C) | H250 | H224 | |||
EUH018 | H242 | ||||
EUH029 | |||||
Explosivity | EUH001 | H200 | |||
EUH006 | H201 | ||||
EUH019 | H202 | ||||
EUH044 | H203 | ||||
H204 | H205 | ||||
H240 | |||||
H241 | |||||
Oxidising properties | H242 | ||||
H270 | |||||
H271 | |||||
Reactivity with water | EUH014 | ||||
This categorisation system relates to occupational exposure limits (OELs) as well. An OEL being a limit level of exposure (so the product of hazard × exposure, see Sect. 26.2), it needs some explanation as to how it is used in the categorisation of just hazards. Although the reason for categorisation is to determine a limit for maximal exposure for each individual substance is not feasible, it is still an ambition. For several substances, mainly those to which many workers and the general public may be exposed, reliable occupational exposure limits (OELs, see Sect. 26.7.2) have been determined. If these OELs are related to other characteristics of those substances, the OELs could virtually be put into the categorising systems. By doing so, the categories of the system could be provided with ‘approximate OELs’.
As an example Table 26.5 gives per category about 10 active substances that have been categorised following Table 26.4, with expert interpretation.
Table 26.5
Hazard categorisation of substances according to the model described in this section: some examples per categorya
Category (hazard class) | Examples of active substances and excipients |
|---|---|
1 | Aluminium magnesium silicate, borax, ethanol, magnesium oxide, paracetamol, peppermint oil, sodium citrate |
2 | Acetylsalicylic acid, aspartame, caffine citrate, isoniazide, lidocaine, nitrazepam |
3 | Butylhydroxytoluene, clioquinol, cocaine hydrochloride, hydrocortisone acetate, phenytoin, omeprazole, salicylic acid, tetracycline hydrochloride |
4 | Atropine sulphate, boric acid, ethinylestradiol, lithium carbonate, testosterone propionate, thioguanine, tretinoin |
5 | Coal tar, colchicine, ganciclovir, methotrexate, mitomycin, paclitaxel, vincristine |
26.3.5.2 Categorisation by NIOSH
The National Institute for Occupational Safety and Health (NIOSH) of the United States has developed a categorisation system for ‘hazardous substances’. NIOSH defines hazardous pharmaceutically active substances using six criteria:
1.
Carcinogenicity
2.
Teratogenicity or other developmental toxicity
3.
Reproductive toxicity
4.
Organ toxicity at low doses in humans (<10 mg/day) or in animals (<1 mg/kg/day)
5.
Genotoxicity
6.
Structure and toxicity profile of new active substances that mimic existing active substances determined hazardous by the above criteria
NIOSH reviews each active substance on an individual basis and does not group them into classes. The review process is accounted for in [29], from which it can be concluded that:
Safe handling recommendations from the manufacturer of a medicine is followed without requiring further review.
Peer reviewers and stakeholders are independently reviewing the status of specific active substances.
NIOSH experts made the final determination; some scientific principles that are followed in that process are given in [8].
Draft conclusions are published for public review.
While the majority of the hazardous active substances in the NIOSH list are antineoplastics, active substances from other classes are included, such as phenytoin and tacrolimus [8]. As part of the categorisation some reference is given about which hazard is the main reason for being included in the list. This NIOSH list is updated regularly which is a great advantage, however as said only the most hazardous substances are included.
26.3.6 Categorisation of Hazards of Products
Categorisation of pharmaceutical preparations (within the field of occupational safety and health seen as mixtures) into hazard categories is relevant because exposure to it can occur in practice. This happens for example when dissolving a mixture of freeze-dried substances or by the crushing of tablets for patients who cannot swallow.
Customising H-statements from substances to products though is not simple. The GHS sets a calculating method for fixing the health hazard of mixtures of substances. The hazard of a mixture depends on the type of hazard and how the concentration of the substances affects the hazardous properties in the preparation. The GHS gives per separate H-statement, if relevant, on how to determine whether the H-statement is maintained, changes or expires depending on the concentration in the mixture. For instance for a property such as corrosiveness, a 10 % content in a mixture may lead to a tenfold decrease of hazard. But for a property such as mutagenicity, although the content in a mixture may be only 0.1 %, the hazard may be classified as large as with a 100 % content [30].
26.4 Exposure Routes and Protection
Different routes may lead to exposure to hazardous substances at the workplace:
Inhalation
Eye contact
Skin contact; primarily with the substance or secondarily via contaminated surfaces
Ingestion
Injection
26.4.1 Inhalation
When an individual breathes in polluted air, any substance may enter the respiratory tract causing direct harm to the respiratory system and indirect harm due to uptake via ingestion. Especially sensitising substances may require attention. As airways and the lung cannot be closed off, only ventilation (exhaustion) and filtration of inhaled air remain as protective measures, such as working in safety cabinets and wearing masks with air filters (respirators).
Ventilation, at which the air that is contaminated with the substance is carried away from the operator, reduces the exposure. Filtration may be necessary to prevent the withdrawn air from contaminating people elsewhere. Ventilation or exhaust can be achieved with the following measures of increasing effectiveness; also increasing containment is attained:
Ventilation of the work space
Use of powder exhaust unit or a fume cupboard
Use of safety cabinet
Use of isolator
Powder and vapour (fume) exhaustion is the first measure to protect the respiratory system of the operator against the hazards of substances. See Sect. 28.3 for a discussion about the equipment and its effectiveness. Section 27.5.1 goes into the air handling (HVAC) of premises when the product should be protected from the operator (with aseptic processes) as well as the operator from the product.
For protection of the airways to inhalation of substances, respirators can be used. Effective respirators however are not pleasant to wear, as the worker never breathes freely and they may be rather heavy as well. Many mouth masks are provided with an in- or exhalation valve or both which eases breathing, see Fig. 26.3.


Fig. 26.3
Examples of FFP2 disposable respirators
Respiratory protection products or respirators are classified on the basis of the achieved applied protection factor (APF). This is the factor by which the exposure by inhalation is reduced as the protection equipment is used in the required manner. Dust filters are positioned in three classes: FFP1, FFP2 and FFP3, usually called P1, P2 and P3 respectively. They provide protection against powders or aerosols or both.
Respirators should comply with national or European standards such as EN 143 for Particulate filters and EN 149 for Filtering half masks to protect against particles. Distinction has to be made between suitability for solid or liquid particle filtration.
FFP1-filters have an APF of 4 for solids, FFP2-filters have an APF of 10 and FFP3-filters have an APF of 20. Quarter masks (which cover a quarter of the face), half-face masks and full-face masks can be discerned. The half- and full-face masks can, if provided with a combined filter for both gas and dust, attain an APF of 40. In addition, quarter or half-face masks if provided with filters for both gas and dust have an APF of 10 for liquids. Full-face masks have an APF of 20 for liquids.
In pharmacy practice employers sometimes choose to provide respirators instead of buying safety cabinets or isolators. This is against the general principles of risk mitigation (see Sect. 26.7.1) and may only be accepted if preparation scarcely takes place. Some specific situations however rely on personal respiratory protection by respirators:
As an additional measure to working in a safety cabinet with solid (powdered) substances of the highest hazard category (which is 4 or 5 depending on the model) for example with the preparation of capsules
Processing heavily corrosive substances
In case of calamities
It is not necessary to wear a respirator when working in an isolator except when the containment is breached for example during the maintenance operations.
26.4.2 Eyes
With regard to eyes corrosive substances are most feared. The wearing of safety glasses can protect eyes rather well. The glasses should fit well and protect the eyes completely. Wearing safety glasses is in any case necessary when:
Operating the ampoules machine
Working with glass equipment under pressure
Filling out corrosive substances
Cleaning up spilt corrosive substances
Wearing of safety glasses is minimally indicated when the substance bears specific H-statements, see Table 26.4, row ‘Acute toxicity’: H314, H318 and H319.
26.4.3 Skin
Corrosive substances are most feared because of their direct harmful effect on the skin. Antineoplastics are most feared with regard to absorption via the skin. The skin of operators may be primarily contaminated by the substance, but also secondarily via contaminated surfaces. Contamination of surfaces is very difficult to avoid, so potentially also contamination of skin of operators, cleaners and even staff who are working in adjoining areas (see further Sect. 26.5.4).
The wearing of the appropriate clothing and gloves can protect the skin rather well. Proper cleaning should diminish the surface contamination and thus the secondary exposure of the skin.
26.4.3.1 Protective Clothing
Clothing may protect the body from hazardous substances. The risk for dermal exposure depending on personal clothing was investigated [31], and can be summarised as Table 26.6; a low score is aimed at.
Table 26.6
Hierarchy in dermal exposure risk with clothing
Personal cloth protection | Score |
|---|---|
No clothing | 1 |
Woven clothing | 0.3 |
Non-woven clothing-permeable | 0.1 |
Non-woven clothing impermeable | 0.03 |
Corporate clothing that covers the clothes should always be worn during preparation processes, and should be changed according to a fixed regime: for example, daily and immediately after spillage. Long hair should be bound together in order to avoid contaminating the product and causing an accident with rotating equipment. Wearing hair and possibly beard caps is part of the clothing regime.
In aseptic processes in pharmacy specific clothing is required to protect the product against micro-organisms from the operator (see Sect. 31.3.3). This may include the wearing of non-shedding suits or coats (depending on the classification of the environment), hair cover, shoe covers or dedicated clean room shoes, gloves, and a respirator covering the nose and mouth.
If also protection from substances is required it is recommended to wear suits or coats made of polyethylene-coated polypropylene (which is nonlinting and non-absorbent). This material is recognised to offer better protection than polypropylene against many antineoplastic substances. The suits must have closed fronts, long sleeves, and elastic or knit closed cuffs. They must be disposed after each use. Moreover, disposable sleeve covers are recommended to protect the wrist area and be removed after the task is complete.
26.4.3.2 Gloves
Wearing gloves for protection of the skin is increasingly common practice with pharmacy preparation. In the risk assessment model of Sect. 26.7.3 it even replaces any effort of estimation of skin exposure. Gloves are minimally indicated when the substance bears specific H statements, see Table 26.4, rows Irritation (skin, respiratory tract and/or eyes) and Sensibilisation (skin and or respiratory tract).
Gloves are available made from different material and having different thickness. They must comply with European standards (for instance [32]). The choice for a particular type depends on:
The mechanical properties of the glove (abrasion resistance, flexibility)
The chemical properties (resistance to the substance from which protection is required)
The permeability of the material for the substance from which protection is required
Substances potentially penetrate gloves, thereby exposing the skin. Specific studies assessed dermal exposure to antineoplastics with regard to the use of gloves [33–38]. The permeability of the gloves is expressed as breakthrough time, the time that a substance needs to pass through the polymer layer. Permeation is related to a variety of factors, such as glove composition, glove thickness, exposure period, and the physical characteristics of the substance [39–41]. Due to the large number of substances and kinds of gloves, only limited data on permeation of specific substances are available.
In practice, for pharmacy preparations the required gloves are of natural rubber (see Sect. 24.2.4) or thick plastic (polyvinyl chloride or polyethylene, see Sect. 24.2.3) if working with corrosive substances. Gloves of thinner material (latex, nitrile rubber, polyvinyl chloride) may be suitable for working with other substances. People who are allergic to natural rubber (latex) can try gloves of nitrile butadiene rubber, vinyl or neoprene.
Gloves are expected to be more permeable as they come into contact with alcohols, for example during disinfection of the workplace. However some authors investigated permeation of antineoplastics when using alcohol and did not find increased permeation with the investigated gloves. Permeability of gloves to selected antineoplastics after treatment with ethanol or isopropyl alcohol is given in [42].
A gloving procedure should take into account:
Glove material and thickness; suppliers of gloves with a CE label supply on suitability for types of substances, protection time (if no other damage has occurred).
Change time, this is the time that gloves will be changed even if no damage has occurred; 30 min is often taken for this.
Inspection; the worker has to be aware of and actively search for any damage that may occur; any damage should lead to changing the gloves.
Single or double gloving; double gloving increases barriers but diminishes precise feeling.
The outcome of these considerations may differ. For handling antineoplastics in many countries in Europe single gloving is the standard in practice, which has to be warranted by frequent change and frequent inspection. The European Society of Oncology Pharmacy [43] seems to prefer double gloving, however without being explicit about the other variables (changing time, thickness, frequency of inspection). NIOSH recommends [44] double gloving when working with NIOSH ‘hazardous substances’ (see Sect. 26.3.4: this represents a high toxicity class) as well as a changing time of 30 min.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree