abnormally raised triglyceride,
Excess dietary calories are stored as fat in adipose tissue. Intra-abdominal (visceral) fat is most closely associated with the metabolic and atherogenic consequences of obesity. The association between central obesity and atherothrombosis is related to enlarged visceral adipocytes. These adipocytes secrete numerous inflammatory, pro-atherogenic and procoagulant cytokines such as interleukin-6, tumour necrosis factor α and plasminogen activator inhibitor-1, but secrete reduced amounts of the anti-inflammatory and anti-atherogenic hormone adiponectin. Cytokines produced by fat-laden omental adipocytes enter the portal circulation and influence many aspects of hepatic metabolism.
Pathogenesis of obesity
Food intake is controlled by numerous hormonal, societal, genetic and psychological factors. Obesity usually develops gradually, and results when energy input exceeds output for a prolonged period. A small imbalance between energy intake and exercise or muscular work is all that is required for progressive weight gain. There are polygenic influences that determine who is more susceptible to weight gain. However, the recent epidemic of obesity in the Western world suggests that major environmental factors (reduced activity and dietary changes) are responsible, rather than biological causes.
For a few individuals, obesity arises from hormonal disturbances, or from neurological conditions that lead to behavioural change. Several drugs can produce weight gain, such as antipsychotics, tricyclic antidepressants, corticosteroids, antiepileptics, antihistamines and antidiabetic drugs.
There are two types of adipose tissue: brown adipose tissue is responsible for thermogenesis and white adipose tissue for energy storage. White adipose tissue is a target for insulin and in obese people there is resistance to the action of insulin, leading to accumulation of intracellular fat.
Energy balance is regulated in the hypothalamus, which integrates neural, hormonal and circulating nutrient stimuli, and sends signals to higher centres to trigger feelings of satiety or hunger. The hypothalamus also regulates sympathetic nervous system function (which controls lipolysis for release of fatty acids as an energy source, and also thermogenesis) and pituitary hormones that help to regulate energy expenditure.
The biochemical factors that underlie the regulation of weight are increasingly well characterised (Fig. 37.1). Signals to the hypothalamus that reduce food intake (satiety signals) are provided by a number of hormones produced by the endocrine cells of the gut, adipose tissue and the pancreas. Leptin, which is released from adipocytes, and insulin both signal via specific hypothalamic receptors to indicate the degree of filling of adipocytes and induce the sensation of satiety.
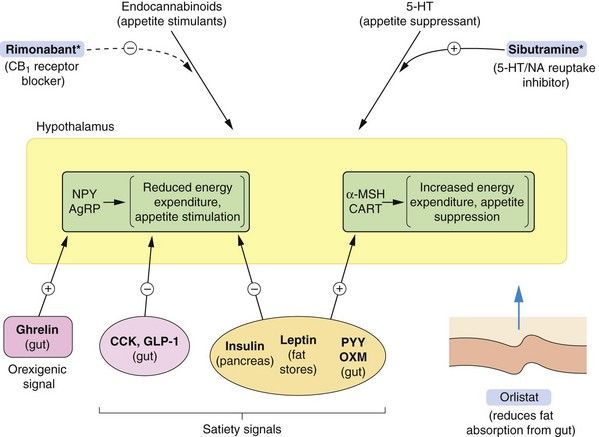
Fig. 37.1 Factors involved in the regulation of food intake.
Feedback loops between the periphery and the brain control food intake. Peripheral signals stimulate or inhibit transmitters in the arcuate nucleus of the hypothalamus (shown in yellow) that control appetite and energy expenditure. The hypothalamus sends signals to higher centres, indicating satiety or hunger. The peripheral orexigenic (appetite-stimulating) signals include ghrelin, released pre-prandially from the gut, and satiety signals include insulin, leptin, peptide YY3-36 (PYY) and oxyntomodulin (OXM). Other hypothalamic signalling mechanisms are described in the text. Endocannabinoid pathways are involved in stimulating appetite, and serotonin acts on the hypothalamus to suppress appetite. *Centrally acting appetite-suppressant drugs have been withdrawn due to unwanted effects, but rimonabant and sibutramine are shown to illustrate mechanisms. Orlistat reduces fat absorption from the gut by inhibiting pancreatic lipase. AgRP, agouti-related protein; CART, cocaine- and amfetamine-regulated transcript; CCK, cholecystokinin; GLP-1, glucagon-like peptide-1; α-MSH, α melanocyte-stimulating hormone; NA, noradrenaline; NPY, neuropeptide Y.
Several gut-derived peptides act as short-term appetite regulators. Ghrelin is released by the stomach pre-prandially, and stimulates orexigenic (appetite-stimulating) peptides. Oxyntomodulin, glucagon-like peptide-1 (GLP-1), cholecystokinin and peptide YY3-36 (PYY) are released from the small intestine and colon in response to the presence of carbohydrates and lipids, and inhibit release of orexigenic peptides in the hypothalamus.
When energy levels are low, ghrelin release and reductions in leptin, insulin and other gut-derived peptides act on the hypothalamus to stimulate key hypothalamic neurotransmitters, including neuropeptide Y (NPY) and agouti-related protein (AgRP). This decreases activity in the melanocortin system which releases signalling by the orexigenic hormones melanin-concentrating hormone (MCH) and orexin (ORX) to stimulate appetite. Following a meal, high plasma concentrations of insulin, glucose, cholecystokinin and leptin stimulate the hypothalamic appetite-suppressing substances pro-opiomelanocortin (POMC) and cocaine- and amfetamine-regulated transcript (CART). These mediators increase α-melanocyte-stimulating hormone (α-MSH) release which inhibits MCH/ORX activity and signal satiety.
Unexpectedly, the circulating concentration of leptin is usually high in obesity. This may reflect the excess fat accumulation and hypothalamic resistance to satiety cues such as leptin.
Other neurotransmitters and hormones that influence appetite include the appetite inhibitors serotonin and dopamine, while cannabinoids, cortisol and growth hormone-releasing hormone stimulate appetite. The role of the endocannabinoid system in regulating appetite has received increased attention in recent years. Acting via CB1 receptors, the natural agonists such as anandamide and 2-arachidonylglycerol have both CNS and peripheral actions. Endocannabinoids in the CNS are released from postsynaptic neurons, and act on presynaptic receptors to inhibit neurotransmitter release. They stimulate appetite by actions at the hypothalamus and nucleus accumbens. Peripherally, endocannabinoids have effects on gastrointestinal motility that reduce satiety signals.
Improved understanding of the biochemical signals that regulate appetite has led to a search for more effective appetite-suppressant drugs. So far there has been little translation into clinical practice.






