GASTROINTESTINAL TRACT STRUCTURE
Substructures and Cells
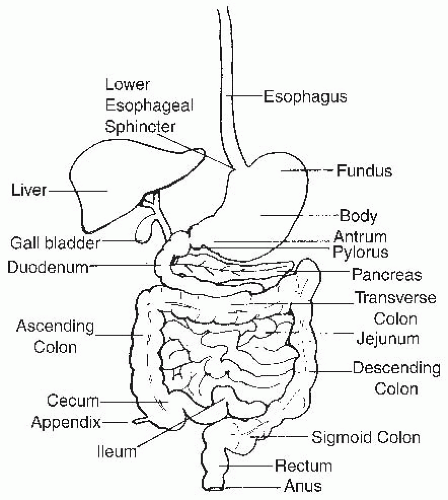
The structure of the GI tract is reviewed briefly, with a consideration of the localization of numerous cells and substructures that are critical for its function. The GI tract consists of four contiguous segments: the esophagus, stomach, small intestine, and colon (
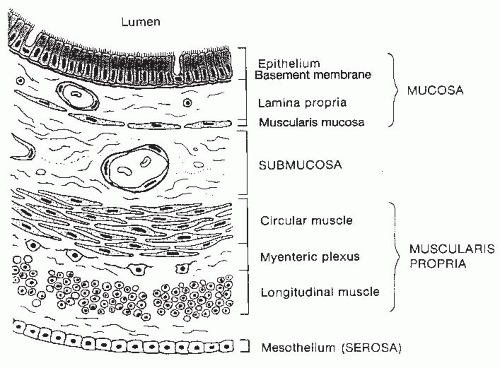
Fig. 42.1). The wall of each segment contains four distinctive layers: the mucosa, submucosa, muscularis propria, and serosa or adventitia (
Fig. 42.2). The mucosa is composed of three distinct layers: the epithelium, lamina propria, and muscularis mucosae. The epithelial layer forms a barrier between the lumen and the underlying tissues. Many of the different region-specific secretory, absorptive, and barrier functions of the alimentary tract are accounted for by differences in the type and distribution of various differentiated epithelial cell populations along the length of the gut. Thus, the epithelium shows the greatest degree of variability among different regions of the GI tract. The lamina propria is a connective tissue space between the epithelium and the thin layer of muscle fibers, the muscularis mucosae, which forms the lower boundary of the mucosa. The lamina propria contains many cells involved in immunologic functions, including immunoglobulin (Ig)-secreting plasma cells, macrophages, and lymphocytes. In addition, abundant lymphoid nodules are present, often extending through the muscularis mucosae into the underlying submucosa. Subepithelial fibroblasts produce collagen and many other extracellular matrix components that underlie the basal lamina of the epithelium. These fibroblasts and the extracellular matrix that they secrete have an important role in regulating cell proliferation and differentiation events within the overlying epithelium.
The mucosal epithelium contains numerous enteroendocrine cells, in addition to cells that serve secretory, absorptive, and barrier functions. The enteroendocrine cells, found in gastric, intestinal, and colonic epithelium, are characterized by their polygonal shape, broad base, and numerous basilar membrane-bound secretory granules. Enteroendocrine cells are joined to other adjacent cells in the epithelium via junctional complexes located near the apical pole. The regulatory peptides or bioamine products stored in the basally located secretory granules are secreted through the basolateral membrane and act through paracrine or endocrine mechanisms as mediators of GI secretion, absorptive function, and motility in response to luminally and/or basolaterally derived signals.
The submucosa extends from the mucosa to the muscularis externa and contains numerous small to moderatesized veins, arteries, and lymphatic channels surrounded by connective tissue. Ganglion cells and autonomic nerve fibers of the Meissner plexus are also found in the submucosa. Fibers of this submucosal plexus together with the myenteric plexus form the enteric nervous system (ENS), which regulates and coordinates numerous intestinal functions, including motility. Additionally, scattered lymphoid aggregates or nodules may be found in this layer of the gut wall.
The muscularis propria is organized into two layers of muscle: an inner circular layer, in which muscle cells circle the intestine, and an outer longitudinal layer, in which muscle cells run parallel with the long axis of the intestine. In the upper esophagus, skeletal muscle fibers interdigitate with smooth muscle fibers, whereas the muscularis of the remaining alimentary tract is composed entirely of smooth muscle.
Esophagus
The adult esophagus is approximately 25 cm long and extends from the posterior oropharynx at the level of
the cricoid cartilage to just below the diaphragmatic hiatus, where it enters the stomach at the esophagogastric junction. The esophageal mucosa is lined with a thick incompletely keratinized stratified squamous epithelium that provides protection against abrasion during passage of a swallowed food bolus and against refluxed stomach acid. The lamina propria contains occasional lymphoid aggregates and mucosal glands that secrete neutral mucus. Submucosal glands that secrete acidic mucus extend through the lamina propria and muscularis mucosae and are most abundant in the upper half of the esophagus.
In the upper esophagus, skeletal muscle fibers blend with the smooth muscle fibers found throughout the rest of the esophagus. The upper esophageal sphincter consists of a thickened band of oblique muscle. These skeletal muscle fibers are under voluntary control and are involved in regulating the initial passage of a swallowed bolus into the upper esophagus. The remaining smooth muscle of the muscularis is innervated by parasympathetic fibers originating from the vagus nerve. A thickened band of circular smooth muscle adjacent to the esophagogastric junction forms the lower esophageal sphincter. Contraction of this specialized region of smooth muscle, coupled with the abrupt angulation of the esophagus as it passes through the diaphragmatic hiatus, where it joins the gastric cardia, provides a mechanism for preventing reflux of the acid contents of the stomach into the esophagus.
Stomach
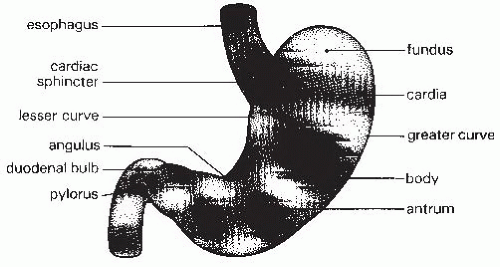
The stomach is an asymmetric organ that extends from the gastroesophageal junction in the cardia to the duodenum (
Fig. 42.3). The upper portion of the stomach that lies under the left hemidiaphragm is called the fundus. The gastric body comprises the largest portion of the stomach and extends to the angularis, where the stomach abruptly bends. The gastric antrum lies between the angularis and the pylorus. The pyloric sphincter is a round band of muscle that forms the opening of the stomach into the duodenum. The flat glandular mucosa of the stomach changes to the villus epithelium seen in the duodenum at the pylorus.
The entire stomach is lined by a simple columnar epithelium. The mucosa contains numerous invaginating gastric pits or foveolae that form glands at their bases. Each glandular unit is composed of three regions: the upper pit region, lined by surface mucus-secreting cells; a narrow isthmus or neck, containing the proliferative zone, and many immature undifferentiated cells and mucous neck cells; and a basilar gland that contains three cell types—parietal cells, chief cells, and enteroendocrine cells. The majority of the gastric body and fundus is lined by oxyntic mucosa, consisting of fundic-type glands responsible for secretion of acid (H+), pepsinogens, and intrinsic factor. These glands contain abundant parietal cells in the upper half of the gland. Chief cells predominate near the base of the glands in fundic-type mucosa. The cardiac glands, found in the first 3 to 4 cm adjacent to the esophagogastric junction, are primarily mucus-secreting glands with few parietal or chief cells. Pyloric glands in the prepyloric antrum are coiled and are remarkable for their fairly long foveolae and increased population of enteroendocrine cells.
Surface mucous cells form a uniform population of columnar epithelial cells lining the surface mucosa and gastric pits. These cells secrete a glycoprotein-rich neutral mucous layer that protects the epithelium from the acid environment of the stomach (
1). Surface mucous cells are constantly shed into the gastric lumen and are replaced by replication of undifferentiated cells within the neck or isthmus region of each gastric gland that differentiate during migration up the foveola and onto the gastric mucosal surface. Mucous neck cells are also present in the neck of the gland. These differ from the surface mucous cells in that their mucous granules are larger, they contain acidic glycoproteins compared with the neutral glycoproteins of the surface mucous cells, and although they secrete mucous, they derive from the stem cell precursors for surface mucous, parietal, chief, and endocrine cells (
2) and likely respond to signals from the mesenchyme, possibly from myofibroblasts (
3).
Parietal cells secrete hydrochloric acid and are located in the middle and basilar portions of the gastric glands. These cells are large with clear or acidophilic cytoplasm and abundant mitochondria. These cells have welldeveloped intracellular canaliculi containing a microvillus border that greatly expands the apical surface available for acid secretion. Receptors for histamine, gastrin, and acetylcholine are located at the basolateral surface and regulate parietal cell secretory function. Hydrogen/potassium (H
+/K
+)-adenosine triphosphatase (ATPase), the enzyme that secretes H
+ into the lumen, is located on the canalicular membrane. Intrinsic factor, a binding protein for
vitamin B
12, is secreted by parietal cells. In addition, parietal cells play a role in the regulation of gastric mucosa cell lineage differentiation. The growth factors transforming growth factor (TGF)-α, heparin-binding epidermal growth factor-like growth factor, and amphiregulin as well as the morphogen sonic hedgehog (a peptide involved in gastric cellular growth an differentiation) are produced by parietal cells (
2,
4).
Chief cells or zymogenic cells are found near the base of the gastric glands. These cells contain an extensive basilar rough endoplasmic reticulum and supranuclear zymogen granules, reflecting their role in the production of pepsinogens and other proteases. Pepsinogens are synthesized and secreted by these cells into the gastric lumen. Hydrochloric acid in the lumen catalyzes the conversion of the proenzyme pepsinogen to the active pepsins that begin digestion of proteins to lower-molecular-weight polypeptides.
Enteroendocrine cells are most abundant in the prepyloric antrum and secrete many different neuropeptides and regulatory molecules that are discussed later. These cells are classified as open or closed cells. Open cells have apical membranes that are in contact with the lumen, whereas closed cells are not in contact with the lumen. Gastrin-secreting G cells predominate in the antrum (an example of open endocrine cell), enterochromaffin cells (ECs) are found throughout the gastric mucosa and secrete serotonin and either substance P or motilin, glucagon-secreting A cells are found in the proximal third of the stomach, and somatostatin-secreting D cells (an example of a closed endocrine cell) can be found in both the upper third of the stomach and the antrum but not in the midstomach. This complex web of enteroendocrine signals is important in integrating responses to both luminal conditions and basolateral signals.
Layers
The stomach has four tissue layers. The first of the layers is the mucosa, which is lined by the epithelial cells outlined in the preceding and also contains lamina propria and a thin muscular layer called the muscularis mucosae. The layer beneath the mucosa is the submucosa. This is a connective tissue layer that contains blood vessels, lymphatics, and nerves. The next layer is the muscularis propria, which is comprised of three muscle layers: the oblique muscle, the circular muscle layer (which becomes the pyloric sphincter), and an outer longitudinal muscle. The final layer is the serosa.
Small Intestine
The small intestine extends from the gastric pylorus to the ileocecal valve and is divided into three regions: the duodenum, the jejunum, and the ileum (
5).
Duodenum
The duodenum is approximately 30 cm in length and is fixed in place, molded around the head of the pancreas. Histologically, the duodenum is characterized by the presence of abundant submucosal Brunner glands that secrete alkaline mucus. The first portion of the duodenum, known as the bulb, is attached to a mesentery that is folded onto the posterior wall of the peritoneal cavity. The second (descending), third (transverse), and fourth (ascending) portions of the duodenum are retroperitoneal in location. Bile and pancreatic secretions enter the second portion of the duodenum from the common bile duct at the ampulla (papilla) of Vater. The junction of the duodenum and the jejunum is defined by the position of the ligament of Treitz, where the duodenum reenters the peritoneal cavity. No change occurs in the histologic appearance of the small intestine at this transition.
Jejunum and Ileum
The jejunum and ileum are mobile because of their attachment to an extensive mesentery. The proximal two fifths of the small intestine beyond the ligament of Treitz are defined as jejunum, whereas the distal three fifths are ileum. The jejunum has a larger diameter, more prominent folds, and longer villi than the ileum. The ileum is characterized by the presence of abundant lymphoid follicles (Peyer patches) in the submucosa.
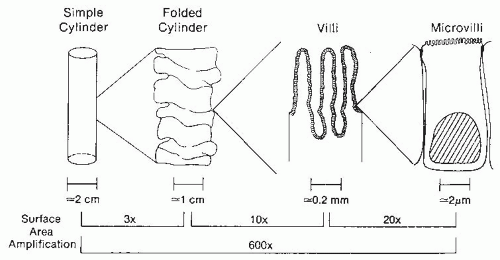
The length of the jejunum and ileum in adults ranges from 320 to 846 cm. Several structural features of the small intestine amplify the mucosal surface area available for nutrient absorption to more than 200 m
2, which is larger than a doubles tennis court (
Fig. 42.4). Magnification of the surface area is achieved by a series of folds and invaginations. First, the cylinder of the intestine is heaped up into circular folds (plicae circulares) involving both submucosa and mucosa. These folds are particularly prominent in the jejunum. Second, the mucosal surface is further expanded by the presence of numerous villi, long fingerlike projections of mucosa containing an arteriole, vein, and central draining lacteal. Third, the apical surface of each small intestinal epithelial cell along the villi is covered by microvilli, providing thousands of hills and valleys for surface expansion. The presence of folds, villi, and microvilli increase the surface area to 600 times that of the surface area present in a simple cylinder.
Epithelium
The simple columnar epithelium that lines the small intestine is composed of four principal differentiated cell types—absorptive enterocytes, goblet cells, Paneth cells, and enteroendocrine cells. Cells are joined to adjacent cells by junctional complexes that regulate the paracellular movement of fluid and macromolecules (see fluid and electrolytes section). Absorptive enterocytes are responsible for digestion of dipeptides, tripeptides, and disaccharides, and for nutrient absorption. The microvilli of absorptive enterocytes are supported by a central core of actin filaments that join with a dense terminal web of actin and myosin filaments oriented parallel to the apical surface of the enterocyte. The apical surface is covered by
a glycoprotein-rich glycocalyx. Many enterocyte-encoded proteins important for digestive function are present at the apical surface, including dipeptidases, disaccharidases, enterokinase, and intestinal alkaline phosphatase. Goblet cells are flask-shaped cells with large apical vesicles that store and secrete mucus. Mucus secreted by the goblet cell forms a viscous gel that functions both as a lubricant and to protect the surface epithelium against adherence of invading pathogens. Goblet cells also secrete small cysteine-rich proteins (trefoil factors) that participate in host defense. Paneth cells reside at the base of the intestinal crypts and produce proteins involved in antibacterial defenses, including lysozyme and a variety of defensins.
Most enteroendocrine cells contain a large number of neuroendocrine mediators (see the section on GI hormones) (
Table 42.1). The distribution of individual enteroendocrine cell subpopulations within the epithelium differs along the length of the small intestine. Although enteroendocrine cells arise from the same stem cell as the other differentiated cell types found in the small intestine, they have a much longer half-life than enterocytes or goblet cells. Thus, their migration onto and along the intestinal villi is uncoupled from the migration of the other epithelial cell types in the intestine. A small population of enteroendocrine cells do exist that differ from other enteroendocrine cells in that they do not contain secretory granules. An example is the brush cell in mice, which produces endogenous opioids as well as uroguanylin (a trypsin-resistant hormone that may act to increase bicarbonate [HCO
3–] secretion) and express Trpm5, a molecule required for signal transduction in taste cells. It is unclear how these cells regulate digestion in humans (
6).
Renewal
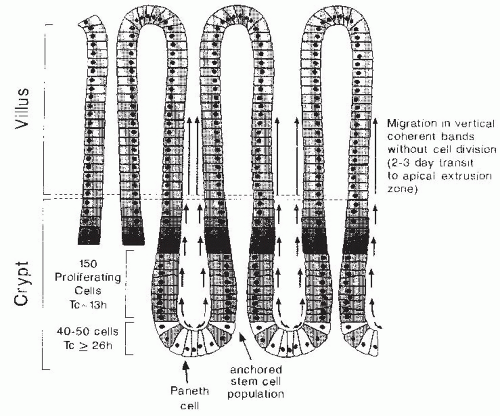
Under normal physiologic circumstances, cells within the intestinal epithelium are continuously and rapidly replaced by migration of cells onto the villus from several adjacent crypts of Lieberkühn or intestinal glands (
Fig. 42.5). The four principal differentiated cell types of the small intestinal epithelium are all derived from multipotent stem cell(s) located near the base of each intestinal crypt (
7). These crypt stem cells divide rarely to produce a daughter stem cell (self-renewal) as well as a more rapidly replicating transit cell (
8). Transit cells, in turn, undergo four to six rapid cell divisions in the proliferative zone located in the lower half of each crypt, and their progeny subsequently differentiate during a bipolar migration away from this zone. Goblet cells and enterocytes undergo terminal differentiation as they are rapidly translocated upward from the zone of proliferation to the apical extrusion zone (a process lasting 48 to 72 hours) located adjacent to the villus tip, where they undergo apoptosis and are sloughed into the lumen. Paneth cells arise during downward migration to the crypt base, and enteroendocrine cells differentiate during migration from the zone of proliferation in either direction. Cell renewal, migration, and differentiation are interrelated processes that are regulated at multiple levels.
Layers
The small intestine is similar to the stomach in that it has four layers: mucosa, submucosa, muscularis propria, and serosa. However, some differences exist. The serosal lining is thinner than the stomach and as it transitions to the small bowel, it becomes continuous with the mesentery. The muscularis propria only contains two muscle layers (the outer longitudinal layer and the inner circular layer) compared with three in the stomach. Between these two layers is the myenteric nerve plexus. The submucosa is similar, but with more prominent vascular structures for absorption. The mucosa is also similar with epithelial cell layer, lamina propria, and a thin layer of muscle called the muscularis mucosae.
Colon
Structure
The colon is approximately 100 to 150 cm in length, extending from the ileocecal valve to the proximal rectum (see
Fig. 42.1) (
9). The colon consists of the cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, and sigmoid colon. The terminal ileum enters the cecum on its posteromedial border at the ileocecal valve. The cecum is a large blind pouch approximately 7.5 to 8.5 cm in diameter that projects from the antimesenteric side of the ascending colon. The appendix extends from a narrow opening in the base of the cecum. The diameter of the colon diminishes progressively; the sigmoid colon is approximately 2.5 cm in diameter and is the narrowest portion of the colon. The omentum is attached to the transverse colon on its anterior superior edge. The ascending colon, descending colon, rectum, and posterior surface of the hepatic and splenic flexures are fixed retroperitoneal structures and therefore lack a complete serosal layer. The cecum, transverse, and sigmoid colon are intraperitoneal and are covered by a complete serosal layer.
Three principal differentiated epithelial cell types are present in the adult colonic epithelium: absorptive colonocytes, goblet cells, and enteroendocrine cells. As found in the small intestine, all these cell lineages appear to be derived from a common epithelial stem cell precursor. Undifferentiated cells, replicating cells, and enteroendocrine cells predominate near the base of each colonic gland (crypt). Cells belonging to each of the principal cell lineages differentiate as they migrate away from the zone of proliferation toward the surface epithelium. The average life span of goblet cells and absorptive cells, from their birth deep in the crypt to the point at which they are sloughed into the lumen, is approximately 6 days. As in the small intestine, some enteroendocrine cell subtypes appear to have a much longer life span than goblet cells or absorptive colonocytes.
As absorptive colonocytes differentiate during their migration up the crypt, they develop short microvilli and clear apically oriented vesicles containing a fibrillar glycoprotein-rich secretory product that may contribute to a glycocalyx. These apical vesicles are lost, and microvilli elongate and increase in number as the maturing absorptive cells emerge onto the surface epithelium. At this point, alkaline phosphatase activity appears on the brush border and the basolateral membranes have acquired a considerable amount of sodium (Na+)/K+-ATPase activity, reflecting their function in water and electrolyte transport.
Many different enteroendocrine cell types are found within the colonic epithelium, including L cells, which contain both enteroglucagon and peptide YY (PYY); cells that secrete only PYY; EC1 cells, which secrete serotonin, substance P, and leu-enkephalin; pancreatic polypeptide (PP)-secreting cells; and rare somatostatin-secreting cells. Enteroendocrine cells are more numerous in the appendix and the rectum than in the rest of the colon.
Layers
The inner circular muscle fibers form a continuous layer around the colon. The outer longitudinal smooth muscle fibers are condensed into three bands (taeniae coli) equidistant around the circumference of the colon. Haustra are the bulging sacculations that form between adjacent taeniae coli. The serosa is a mesothelially derived cell layer that covers the peritoneal aspects of the colonic wall. Therefore, regions of the ascending colon, the descending colon, and the rectum that do not lie within the peritoneal cavity have no outer serosal layer.
Appendix
The appendix is similar in histologic organization to the rest of the colon. The mucosa of the appendix consists of deep folds lined with a columnar epithelium forming simple tubular or forked glands. This epithelium contains abundant goblet cells and enteroendocrine cells. Numerous lymphoid nodules are found in the lamina propria. The normal histologic architecture of the adult appendix is often replaced by fibrous scar tissue as a result of subclinical bouts of appendicitis.
Rectum
The rectum is approximately 12 to 15 cm in length and extends from the sigmoid colon to the anal canal following the curve of the sacrum (see
Fig. 42.1). The rectal wall consists of mucosal, submucosal, inner circular, and outer longitudinal muscular layers. No serosal layer exists in the rectum. The anal canal is approximately 3 cm long. The anal verge is the junction between anal and perianal skin. Anal epithelium (anoderm) lacks hair follicles, sebaceous glands, and sweat glands. The dentate line is the true mucocutaneous junction located just above the anal verge. A 6- to 12-mm transitional zone exists above the dentate line, where the squamous epithelium of the anoderm becomes cuboidal and then columnar epithelium.
VASCULATURE
Blood and lymphatic vessels provide the transportation system for delivering absorbed nutrients to other body tissues (
10). In addition, the arterial blood supply provides nutrients to the alimentary tract itself. In the small intestine, each villus contains a single arteriole that breaks into a capillary network at the villus tip before anastomosing with a draining venule. Each villus contains a lymphatic vessel (lacteal) that drains into a submucosal plexus connected to larger lymphatics. In the colon, arterioles pass between crypts to the epithelial cell surface and form a network of capillaries around the crypts. Lymphatic vessels in the colon do not extend higher than the base of the crypts.
Blood from the small intestine and colon drain into the portal vein that delivers absorbed water-soluble nutrients directly to the liver, where these nutrients can be metabolized or released directly into the hepatic veins
and ultimately into the systemic circulation (
11). Bile salts absorbed in the terminal ileum travel through the portal vein to the liver, where they can be secreted back into the small intestine, providing an enterohepatic circulation for bile salt recycling, which is critical for normal bile salt homeostasis and fat absorption. Intestinal lymphatic vessels, which are closely associated with arteries supplying the alimentary tract, carry absorbed fat-soluble nutrients to the thoracic duct, which drains into the left subclavian vein and the systemic circulation.
Adequate intestinal blood flow is critical because it provides the oxygen necessary for intestinal cell survival. Therefore, GI tract blood flow is carefully regulated by metabolic, vascular, and hormonal factors to ensure adequate tissue oxygenation (
12). Food ingestion increases intestinal blood flow and oxygen requirements (
13).
ENTERIC NERVOUS SYSTEM AND MOTILITY
The ENS is able to regulate such complex and diffuse motility functions by its vast network throughout the GI tract. The ENS consists of approximately 100 million nerve cell bodies (neurons) and their processes that are embedded in the wall of the GI tract (
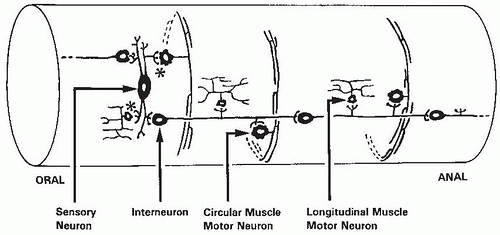
Fig. 42.6). These neurons lie in clusters (ganglia) and are segregated largely into two layers: (a) the myenteric ganglia, which form a continuous plexus between the circular and longitudinal muscle layers of the muscularis propria and extend from the upper esophagus to the internal anal sphincter; and (b) the submucous plexus, which is located in the submucosa and is especially prominent in the small and large intestines. The processes from these ganglia form dense networks and innervate the muscularis propria, muscularis mucosae, epithelium, and other structures. Also, nonganglionated plexuses supply all of the layers of the tubular GI tract accompanying the arteries that supply the gut wall.
The ENS has many different types of neurons (
Table 42.2). Moreover, these neurons may differ in function in different regions of the digestive tract. Excitatory motor neurons innervate the longitudinal and circular smooth muscle and muscularis mucosae. In addition, they innervate enteric endocrine cells and Peyer patches. Secretomotor neurons in the small and large intestine and gallbladder regulate water and electrolyte secretion. In the stomach, they stimulate acid secretion. Interneurons are present in all regions of the gut, but their characteristics vary more than those of other neuron types. They are present as a chain within the myenteric plexus that runs from mouth to anus. Intrinsic reflex pathways that control gut movement, blood flow, and secretion are activated by sensory neurons that respond to mechanical and chemical stimuli and to distention. These neurons are now known as intrinsic primary afferent neurons. Intrinsic primary afferent neurons are multiaxonal and connect to other intrinsic primary afferent neurons, motor neurons, and interneurons. They differ from extrinsic sensory neurons because their responses can be modified by synapses at the cell body.
The ENS is connected to the central nervous system (CNS) by transmission along axons in both directions, from GI tract to brain and from brain to ENS. The connections are largely through the vagus nerve and pathways leaving the spinal cord. Most vagal fibers (75% to 90%) are afferent fibers that interact with neurons in the nucleus tractus solitarius in the midbrain. Because relatively few efferent vagal fibers exist compared with the large number
of ENS neurons, the vagus functions more to initiate activity of the integrated circuits in the ENS rather than to coordinate gut function by direct signaling. Efferent centers in the spinal cord can receive efferent signals from the CNS, which are relayed to the ENS. In addition, the spinal centers can process afferent signals from the gut (
14).
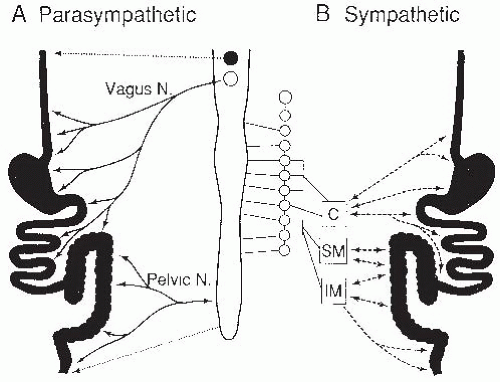
The vagal and spinal components comprise the extrinsic branches of the autonomic nervous system, including the parasympathetic and sympathetic systems (
Fig. 42.7). The striated muscles in the upper esophagus and external anal sphincter are directly innervated by cholinergic fibers, whereas the remaining gut is innervated by a variety of neural mediators, including acetylcholine, gut peptides, and nitric oxide (NO). These preganglionic fibers form synapses with the enteric plexuses, which in turn are connected with smooth muscle, secretory, and endocrine cells. The sympathetic nervous system contains preganglionic connections between prevertebral ganglia and the spinal cord, but the gut itself is innervated by postganglionic connections, mediated largely by epinephrine and norepinephrine. These postganglionic fibers innervate the plexuses of the ENS, as do the parasympathetic fibers, but the sympathetic fibers also directly innervate blood vessels, smooth muscle layers, and mucosal cells.
The sympathetic nervous system affects intestinal secretion, blood flow, and motility. The sensory fibers that accompany the sympathetic nerves (intestinofugal neurons) are primary sensory neurons that are not part of the autonomic nervous system, and are not really “sympathetic” sensory nerves. Sympathetic efferent neurons inhibit motility by decreasing contractile activity and constricting sphincters. These various effects can be relayed along the gut to other regions before returning to the region of the initial stimulus by means of prevertebral ganglia connections. Examples of these inhibitory reflexes include slowing of gastric emptying by acidity or hypertonicity in the upper small intestine.
Intestinal smooth muscle is of the unitary type and is characterized by spontaneous activity, including active tension to stretching, and activity that is not initiated by nerves, but modulated by them. The circular muscle is innervated by both excitatory and inhibitory motor neurons and forms a thick syncytium surrounding the submucosa. Contraction shortens the radius but increases the length of each fiber, and in turn, of the syncytium. In contrast, the longitudinal muscle layer surrounding the circular muscle is thin, is shortened by contraction (with enlarged radius), and is only innervated by excitatory neurons. Electrical slow waves derive from the muscle itself
and trigger action potentials, which lead to contractile activity. Action potentials in intestinal smooth muscle are propagated through gap junctions from cell to cell, creating an electrical syncytium.
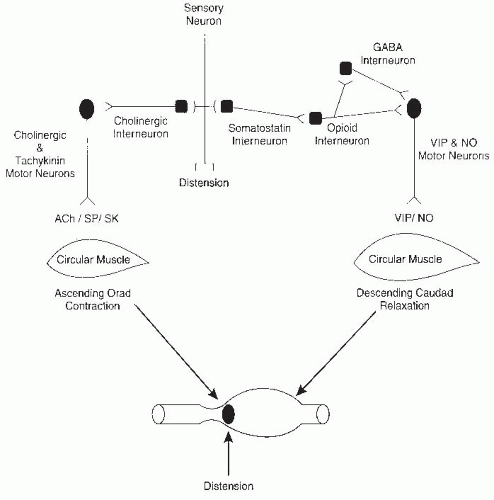
The regulation of peristalsis, the smallest unit of the propulsive reflex, is one of the simplest of the programmed motor activities of the ENS but is still quite complex (
Fig. 42.8). Two components of the reflex, orad contraction and caudad relaxation, combine to move intestinal contents in a caudad direction. The propulsive movement is the end result of contractions and relaxations of the longitudinal and circular external muscles and the muscularis mucosae. The circular muscle has the major role in mixing and propulsion by ring contractions that decrease the diameter of the intestine, whereas the longitudinal muscle creates shortening of the segment by sleeve contractions with little alteration in luminal diameter. Excitatory and inhibitory motor neurons supply the muscle, and inhibitory reflexes modulate these activities by monitoring luminal contents. Multiple chemical mediators are involved in this reflex (see
Fig. 42.8 and
Table 42.1).
Many GI tract motor patterns have been described and involve complicated interactions between a series of stimulatory and inhibitory impulses from the ENS to GI smooth muscle. Intestinal smooth muscle consists of circular and longitudinal muscle layers, so the interaction of muscular contraction between layers determines the pattern of motility. The two most important motility patterns are the migrating motor complex (MMC) and peristalsis, which are programmed by the ENS (
15).
The MMC, the major complex fasting motility pattern in mammals, is cyclic and passes from the stomach to the terminal ileum (
16). The migrating myoelectric complex consists of coordinated activity that empties the stomach and sweeps down the intestine, lasts for 84 to 112 minutes, and is separated into three phases. During phase I, very little motor activity occurs, with only a small amount of forward propulsion. In phase II, irregular contractions occur, and duodenal cross-sectional diameter also increases. This may be to accommodate biliary secretions, which also occurs during this phase. Propulsion occurs during this phase, with rapid propulsion in the transition from phase II to phase III. Phase III lasts only for 5 to 10 minutes of the MMC cycle, but propagates contractions over much longer distances than in phase II, with contractions starting in the gastric body. In addition, a few of the contractions in phase III are retrograde, which reflux duodenal contents and HCO
3– into the antrum of the stomach. This increases the pH in this region of the stomach and may act to protect the mucosa in the fasting state. The maximal frequency of contractions is determined by the slow wave frequency (myocyte cell membrane potential
fluctuations which occur with a certain frequency and along the length of the bowel), which is 11 to 12 contractions per minute in the duodenum and 7 to 8 contractions per minute in the ileum. The functional role of these interdigestive movements is to clear the gut for the next meal. During fasting, the fundus of the stomach is in a state of partial contraction. The pressure created by this partial contraction decreases with a food bolus in response to receptive relaxation (stimulated swallowing) and gastric accommodation (stimulated by gastric distention) (
17).
In the fed pattern, the contractions in the stomach mimic those of phase II of the MMC. This lasts until the stomach is empty and starts 5 to 10 minutes after eating commences. These contractions propel the food distally and proximally in the stomach, ultimately mixing and grinding the food. The amount of time food is in the stomach depends on the number of calories consumed and the amount of fat consumed (
17). In the small intestine and colon, the MMC is replaced by a fed pattern of contraction. This is characterized by intermittent phasic contractions throughout the small intestine. Again, increased fat content increases the amount of time spent in this fed motor pattern. The return of the MMC after feeding is not well understood, but the first MMC contractions may start more distally in the small bowel. It is unclear what signals are involved (
16).
The presence of luminal nutrients can increase absorption by feedback regulation of intestinal motility, called the ileal brake. Fat or carbohydrate in the ileum stimulate the release of PYY, glucagon-like peptide-1 (GLP-1), and possibly oxyntomodulin (OXM) from ileal endocrine cells (
18). These hormones then enter the systemic circulation and inhibit gastric emptying, thereby slowing down small intestinal transit. Thus, this ileal brake mechanism enhances absorption by increasing the contact time between luminal nutrients and intestinal mucosa. Although luminal nutrients in the colon also have some effect on PYY and GLP-1 secretion, they have not been shown to affect small bowel transit times in humans at doses able to alter small bowel transit times when infused in the ileum (
18).
GASTROINTESTINAL HORMONES
The largest endocrine organ in the human body is the GI tract, and the first hormone ever discovered was secretin, a peptide hormone produced in the GI tract. The GI tract hormones are briefly reviewed in this section. The mucosa of the GI tract differs from other endocrine organs in that the endocrine and peptidergic neurons are found diffusely throughout the GI tract (
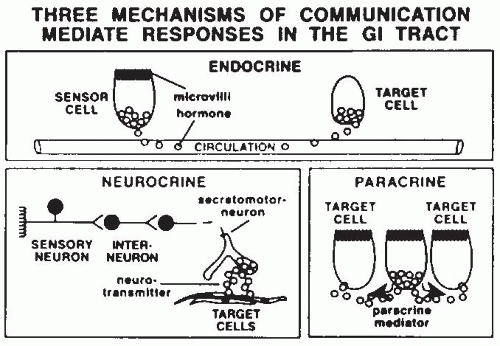
19). These endocrine and peptidergic neurons produce regulatory substances that are critical for the precise coordination of activities necessary to handle a meal. These substances are mostly peptides that communicate by endocrine, neurocrine, and paracrine pathways (
Fig. 42.9; see
Table 42.1), and some of the GI tract hormones can act via more than one communication route. Endocrine peptides are hormones released from sensory cells in the intestine in response to a mechanical or chemical stimuli and enter the bloodstream to act on a distant target organ. Gut neurocrine peptides are produced within the ENS and are located in nerves within the gut itself. Most of these peptides (and their receptors) are also produced by the brain and represent a gut-brain axis. Paracrine peptides (and histamine, an amine) are produced by intestinal cells and act on adjacent or nearby cells. This can occur either by direct cellular extension to other cells, or release of the
peptide (or histamine) into the mucosa (e.g., somatostatin, histamine) or into the intestinal lumen (e.g., monitor peptide, cholecystokinin [CCK]-releasing peptide, trefoil peptides).
The GI tract hormones have multiple effects, both short term in response to a meal, and more delayed on the growth and differentiation of enteric cells.
Table 42.1 lists the better-characterized GI tract hormones. Many of the GI tract hormones that are important in response to a meal are produced by cells in the upper GI tract and act on the components of the upper GI tract (e.g., gastrin, CCK, secretin, motilin, glucose-dependent insulinotropic peptide [GIP], somatostatin, neuropeptide Y [NPY], leptin, ghrelin). All three major macronutrients (protein, carbohydrate, fat) are responsible for the release of these substances. Because the coordination of function in the upper intestinal tract is so crucial, involving the stomach, duodenum, pancreas, and gallbladder, it is not surprising that these sites are most important in the release of GI hormones.
The specificity and coordination of action of GI hormones depend on three major factors: the multiple functions of each hormone, the paracrine actions between neuroendocrine and mucosal cells, and the regulatory functions of the ENS. Most GI hormones have multiple actions and mediate both stimulatory and inhibitory functions (e.g., gastrin, CCK, secretin, GIP, vasoactive intestinal polypeptide [VIP], enkephalins) (see
Table 42.1). Other GI hormones or amines are solely stimulatory (e.g., histamine, motilin, gastrin-releasing peptide [GRP], monitor peptide, CCK-releasing peptide), or inhibitory (e.g., somatostatin, PP). Thus, release of these hormones has the potential to create multiple effects on GI organs coordinated in time. The presence of multiple cells in the mucosa, each possessing receptors to many of the GI hormones, also helps to create specificity of response. For example, in isolated cell systems, CCK stimulates acid production. However, CCK injected into the intact animal does not stimulate acid production because of the greater effect of CCK on the D cell-producing somatostatin, an inhibitor of acid secretion, than on the parietal cell that produces acid. Gastrin, conversely, has the reverse effects on those two mucosal cells, stimulating gastric acid secretion from the parietal cell. In this way, the multiplicity of mucosal-specific cells adds a layer of complexity and control to the presence of multiple hormones present in the mucosa. Finally, the ENS, with its many neuronal connections to mucosal cells, integrates the stimuli controlling GI hormone release. Both preganglionic parasympathetic cholinergic nerves and postganglionic fibers, mediated by neurocrine peptides, are important regulators of the GI response to feeding. In addition, chemosensory neurons detect intraluminal events and regulate mucosal function by intrinsic mucosal reflexes.
Peptide hormones are also involved in appetite regulation (see the chapter on control of food intake and appetite). Hormones such as GLP-1, CCK, PYY, PP, OXM, amylin, insulin, glucagon, and ghrelin interact with the brain in both the hypothalamus and in the brainstem (area postrema) by passage through the blood-brain barrier, acting via vagal-brainstem-hypothalamic pathways, or both. All of these hormones listed, with the exception of ghrelin, result in decreased caloric intake and are considered appetite suppressing. Ghrelin is produced by the endocrine cells in the gastric fundus, and intravenous infusions of ghrelin in lean persons stimulate appetite and consumption of food (
20).
Some peptide hormones are important mitogens for the cells of the intestinal tract. Gastrin stimulates the growth of gastric oxyntic gland mucosa. GLP-1 and GLP-2 are produced in gut endocrine cells. These peptides are released by nutrient ingestion, and regulate cell proliferation and differentiation in the intestine in addition to their role in energy disposal. Insulinlike growth factor-I (IGF-I) is also produced by gut mucosal cells and is a potent trophic factor for intestinal mucosa, principally targeting epithelial cells, endothelial cells, and fibroblast (
21). GLP-1 and GLP-2 also have antiapoptotic activity, thereby enhancing their effect on mucosal growth (
22).