with water and electrolytes, predominantly bicarbonate, which enhances luminal enzyme function by neutralizing gastric acid. The most active enzymes are lipase, amylase, and trypsin. Amylase (α-amylase) hydrolyzes dietary starch into disaccharides and trisaccharides, which are then broken down by enzymes on the brush border to absorbable forms as glucose and maltose. Pancreatic lipase hydrolyzes fat molecules. Bile salts secreted by the liver aid the digestive action of lipase by coating and emulsifying large fat droplets into smaller droplets, thus increasing the overall surface area for lipase to work. Fat hydrolysis results in formation of monomers (two free fatty acids and one 2-monoacylglycerol), which are then absorbed downstream into the lymphatic system by the lacteals. The main proteolytic enzyme, trypsin, is synthesized in the pancreas in an inactive form, as trypsinogen. Following a meal, when the pancreas is stimulated by CCK and cholinergic reflexes, trypsinogen is released from zymogen stores in the acinar cells and is secreted into the duodenum. Once in the small intestine, the intestinal enzyme enteropeptidase activates it into trypsin by proteolytic cleavage. Trypsins then, by the process of autocatalysis, activate more trypsinogen molecules. Once activated, the trypsin breaks down food proteins and peptides (proteins broken down to peptides in the stomach by pepsin) to amino acids, which are then absorbed by active transport systems.
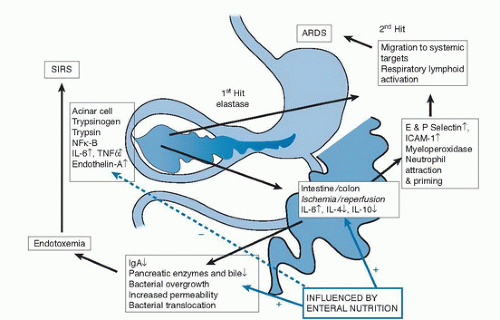
other trypsinogen molecules and autodigestion of the cell (9, 10). Intracellular injury results in generation of a cascade of proinflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-8, IL-15, monocyte chemotactic protein-1 (MCP-1), and IL-18 (11, 12, 13, 14, 15, 16, 17) via activation of periacinar myofibrocytic nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein (MAP) kinase (18). The intense inflammatory response results in arterial constriction with resultant apoptosis, which, in an extreme situation, may lead to pancreatic necrosis. If the inflammation were contained within the pancreatic bed, the disease process would be far less serious. Unfortunately, the cytokines are released into the circulation, and a secondary response commencing approximately 48 hours later leads to the generation of prostaglandin-2, thromboxane, leukotriene B4, and oxygen-derived free radicals within the bronchial and intestinal mucosa that produce cytotoxic lung injury (19, 20).
TABLE 81.1 RANSON SCORE (INCLUDES EQUALLY [1 POINT] WEIGHTED VARIABLES)a | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
TABLE 81.2 COMPUTED TOMOGRAPHY SEVERITY SCORE (BALTHAZAR SCORE)a | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
for pancreatic rest for 48 hours. In most cases, the pain improves, nausea and vomiting resolve, serum lipase and amylase levels drop, the patient feels better, and oral intake can be retested. Traditionally, patients have been started on a clear liquid diet and then advanced to a low-fat soft diet. More recent randomized controlled trials (RCTs) have, however, reported that starting these patients on a low-fat soft diet is as safe as a clear liquid diet, with varying effect on length of hospital stay (36, 37, 38). These findings suggest that after 2 to 3 days of pancreatic rest, patients can be tried on a low-fat soft diet with close monitoring for abdominal pain, nausea, vomiting, or any other complication. Once patients exhibit tolerance to this diet, they can be advanced to a regular diet over next 3 to 4 days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree