32
Nucleotides
OBJECTIVES
After studying this chapter, you should be able to:
![]() Write structural formulas to represent the amino- and oxo-tautomers of a purine and of a pyrimidine and state which tautomer predominates under physiologic conditions.
Write structural formulas to represent the amino- and oxo-tautomers of a purine and of a pyrimidine and state which tautomer predominates under physiologic conditions.
![]() Reproduce the structural formulas for the principal nucleotides present in DNA and in RNA and the less common nucleotides 5-methylcytosine, 5-hydroxymethylcytosine, and pseudouridine (Ψ).
Reproduce the structural formulas for the principal nucleotides present in DNA and in RNA and the less common nucleotides 5-methylcytosine, 5-hydroxymethylcytosine, and pseudouridine (Ψ).
![]() Represent D-ribose or 2-deoxy-D-ribose linked as either a syn or an anti conformer of a purine, name the bond between the sugar and the base, and indicate which conformer predominates under most physiologic conditions.
Represent D-ribose or 2-deoxy-D-ribose linked as either a syn or an anti conformer of a purine, name the bond between the sugar and the base, and indicate which conformer predominates under most physiologic conditions.
![]() Number the C and N atoms of a pyrimidine nucleotide and of a purine nucleoside, including using a primed numeral for C atoms of the sugars.
Number the C and N atoms of a pyrimidine nucleotide and of a purine nucleoside, including using a primed numeral for C atoms of the sugars.
![]() Compare the phosphoryl group transfer potential of each phosphoryl group of a nucleoside triphosphate.
Compare the phosphoryl group transfer potential of each phosphoryl group of a nucleoside triphosphate.
![]() Outline the physiologic roles of the cyclic phosphodiesters cAMP and cGMP.
Outline the physiologic roles of the cyclic phosphodiesters cAMP and cGMP.
![]() Appreciate that polynucleotides are directional macromolecules composed of mononucleotides linked by
Appreciate that polynucleotides are directional macromolecules composed of mononucleotides linked by ![]() -phosphodiester bonds.
-phosphodiester bonds.
![]() Understand that in the abbreviated representations of polynucleotide structures such as pTpGpT or TGCATCA, the 5′-end is always shown at the left and all phosphodiester bonds are
Understand that in the abbreviated representations of polynucleotide structures such as pTpGpT or TGCATCA, the 5′-end is always shown at the left and all phosphodiester bonds are ![]() .
.
![]() For specific synthetic analogs of purine and pyrimidine bases and their derivatives that have served as anticancer drugs, indicate in what ways these compounds inhibit metabolism.
For specific synthetic analogs of purine and pyrimidine bases and their derivatives that have served as anticancer drugs, indicate in what ways these compounds inhibit metabolism.
BIOMEDICAL IMPORTANCE
In addition to serving as precursors of nucleic acids, purine and pyrimidine nucleotides participate in metabolic functions as diverse as energy metabolism, protein synthesis, regulation of enzyme activity, and signal transduction. When linked to vitamins or vitamin derivatives, nucleotides form a portion of many coenzymes. As the principal donors and acceptors of phosphoryl groups in metabolism, nucleoside tri- and diphosphates such as ATP and ADP are the principal players in the energy transductions that accompany metabolic interconversions and oxidative phosphorylation. Linked to sugars or lipids, nucleosides constitute key biosynthetic intermediates. The sugar derivatives UDP-glucose and UDP-galactose participate in sugar interconversions and in the biosynthesis of starch and glycogen. Similarly, nucleoside-lipid derivatives such as CDP-acylglycerol are intermediates in lipid biosynthesis. Roles that nucleotides perform in metabolic regulation include ATP-dependent phosphorylation of key metabolic enzymes, allosteric regulation of enzymes by ATP, ADP, AMP, and CTP, and control by ADP of the rate of oxidative phosphorylation. The cyclic nucleotides cAMP and cGMP serve as the second messengers in hormonally regulated events, and GTP and GDP play key roles in the cascade of events that characterize signal transduction pathways. Medical applications include the use of synthetic purine and pyrimidine analogs that contain halogens, thiols, or additional nitrogen atoms in the chemotherapy of cancer and AIDS, and as suppressors of the immune response during organ transplantation.
CHEMISTRY OF PURINES, PYRIMIDINES, NUCLEOSIDES & NUCLEOTIDES
Purines & Pyrimidines Are Heterocyclic Compounds
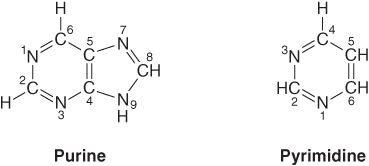
Purines and pyrimidines are nitrogen-containing heterocycles, cyclic structures that contain, in addition to carbon, other (hetero) atoms such as nitrogen. Note that the smaller pyrimidine molecule has the longer name and the larger purine molecule the shorter name, and that their six-atom rings are numbered in opposite directions (Figure 32–1). Purines or pyrimidines with an -NH2 group are weak bases (pKa values 3-4), although the proton present at low pH is associated, not as one might expect with the exocyclic amino group, but with a ring nitrogen, typically N1 of adenine, N7 of guanine, and N3 of cytosine. The planar character of purines and pyrimidines facilitates their close association, or “stacking,” that stabilizes double-stranded DNA (see Chapter 34). The oxo and amino groups of purines and pyrimidines exhibit keto-enol and amine-imine tautomerism (Figure 32–2), although physiologic conditions strongly favor the amino and oxo forms.
FIGURE 32–1 Purine and pyrimidine. The atoms are numbered according to the international system.
FIGURE 32–2 Tautomerism of the oxo and amino functional groups of purines and pyrimidines.
Nucleosides Are N-Glycosides
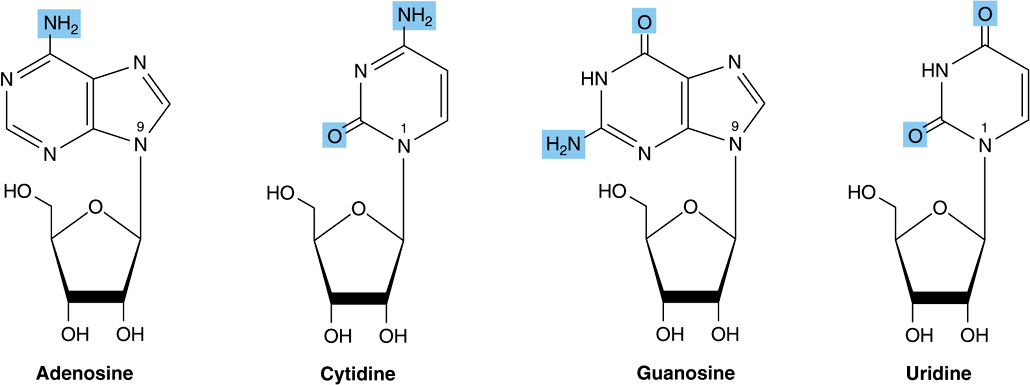
Nucleo sides are derivatives of purines and pyrimidines that have a sugar linked to a ring nitrogen of a purine or pyrimidine. Numerals with a prime (eg, 2′ or 3′) distinguish atoms of the sugar from those of the heterocycle. The sugar in ribonucleosides is D-ribose, and in deoxyribonucleosides is 2-deoxy-D-ribose. Both sugars are linked to the heterocycle by a β-N-glycosidic bond, almost always to the N-1 of a pyrimidine or to N-9 of a purine (Figure 32–3).
FIGURE 32–3 Ribonucleosides, drawn as the syn conformers.
Nucleotides Are Phosphorylated Nucleosides
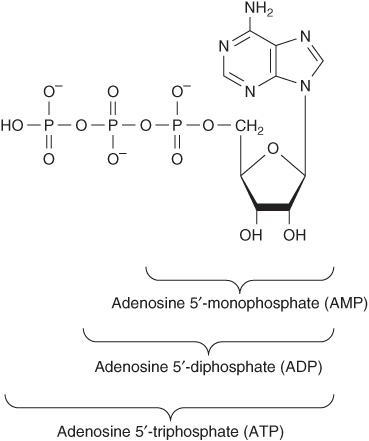
Mononucleotides are nucleosides with a phosphoryl group esterified to a hydroxyl group of the sugar. The 3′- and 5′-nucleotides are nucleosides with a phosphoryl group on the 3′- or 5′-hydroxyl group of the sugar, respectively. Since most nucleotides are 5′-, the prefix “5’-” usually is omitted when naming them. UMP and dAMP thus represent nucleotides with a phosphoryl group on C-5 of the pentose. Additional phosphoryl groups, ligated by acid anhydride bonds to the phosphoryl group of a mononucleotide, form nucleoside diphosphates and triphosphates (Figure 32–4).
FIGURE 32–4 ATP, its diphosphate, and its monophosphate.
Heterocylic N-Glycosides Exist as Syn and Anti Conformers
Steric hindrance by the heterocycle dictates that there is no freedom of rotation about the β-N
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree