Nose, Paranasal Sinuses, and Nasopharynx
Stacey E. Mills
The nasal cavity, paranasal sinuses, and nasopharynx are sites for a wide variety of tumors and nonneoplastic conditions. The large number of diseases affecting these structures is, in major part, a result of the many specialized tissues, each with its own associated aberrations that exist in this region. Basic knowledge of the anatomy and histologic features of this area is of value because many conditions show a strong predilection for a specific anatomic location. For example, angiofibromas and lymphoepitheliomas develop almost exclusively in the nasopharynx, lobular capillary hemangiomas and schneiderian papillomas involve the nasal cavity, intestinal-type adenocarcinomas typically occur in the paranasal sinuses, and olfactory neuroblastomas arise from the superior portion of the nasal cavity.
REVIEW OF ANATOMY
NASAL CAVITY
The nasal cavity is divided into the nares by the nasal septum and, at the anterior extreme, the columella. Each cavity is wedge-shaped, being narrow superiorly and wider inferiorly (1,2). The anterior portion of each nasal cavity is the vestibule (Fig. 21.1). The choana forms the posterior border of each cavity, separating it from the nasopharynx. The nasal turbinates are the lateral walls of each nasal cavity, and the cribriform plate forms the superior boundary. The lower portion of the vestibule is lined by skin containing adnexal structures, including hair. Posterior to the vestibule, the nasal cavity, except for its uppermost portion, is lined by thick, highly vascularized, ciliated columnar epithelium. This mucosa, in conjunction with that of the paranasal sinuses, is often referred to as the schneiderian membrane. Goblet cells may be present in the surface mucosa, and secretions from the seromucinous glands beneath the surface drain into the nasal cavity through small ducts. Areas of squamous metaplasia may occasionally be found, particularly on the anterior surface of the middle and lower turbinates and the anterior nasal septum (1).
The superior one-third of the nasal septum, the superior turbinate, and the cribriform plate are covered with thinner olfactory mucosa. The latter develops at the embryonic stage from the respiratory mucosa. The specialized receptor cells of the olfactory mucosa have neuroendocrine features and grow through the cribriform plate to establish contact with the central nervous system. In infants, the olfactory mucosa is uniform and sharply demarcated; in adults, however, progressive atrophy leads to an irregular, patchy distribution with intervening islands of respiratory epithelium (3).
PARANASAL SINUSES
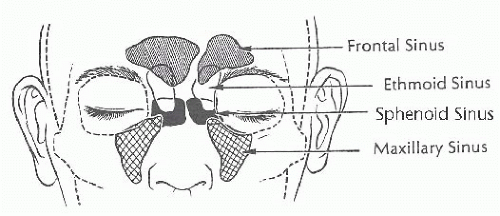
The paranasal sinuses are diverticula of the nasal cavity that extend into neighboring bones (Fig. 21.2). The ethmoid sinuses begin development in fetal life and are well developed at birth. The frontal, maxillary, and sphenoid sinuses, in contrast, are small or rudimentary at birth. The maxillary and sphenoid sinuses develop rapidly during childhood and reach essentially adult configuration after permanent dentition is acquired. The frontal sinuses begin development in childhood and continue to enlarge through puberty. The mucosa of the paranasal sinuses is in continuity with the nasal cavity and consists of identical, pseudostratified, ciliated columnar epithelium. Typically, it is only approximately half as thick as the nasal mucosa, with fewer associated goblet cells and seromucinous glands.
NASOPHARYNX
The nasopharynx is the part of the respiratory passage that lies above and behind the soft palate (Fig. 21.1). It has anterior, posterior, and lateral walls (4). The anterior wall is perforated by the posterior nares (choanae). The posterior wall is an arch
that includes the roof of the nasopharynx; it also comprises the posterior portion against the base of the skull. The posterior wall extends inferiorly to the level of the free border of the soft palate, at which point the oropharynx begins. The ostium of the eustachian tube, located in the lateral wall, is surrounded by a mucosa-covered cartilaginous prominence. Posterior to this ostium is the pharyngeal recess or fossa of Rosenmüller.
that includes the roof of the nasopharynx; it also comprises the posterior portion against the base of the skull. The posterior wall extends inferiorly to the level of the free border of the soft palate, at which point the oropharynx begins. The ostium of the eustachian tube, located in the lateral wall, is surrounded by a mucosa-covered cartilaginous prominence. Posterior to this ostium is the pharyngeal recess or fossa of Rosenmüller.
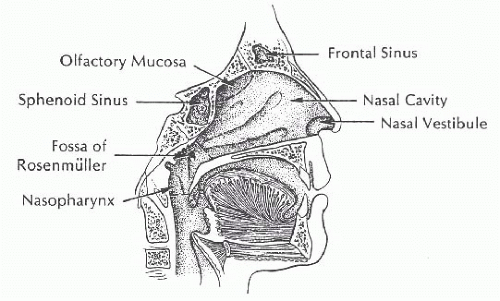 FIGURE 21.1 This midline sagittal section demonstrates the contours of the nasal cavity, limits of the nasopharynx (straight dashed lines), and region of olfactory mucosa (curved dashed line). |
The nasopharyngeal mucosa in the adult has a surface area of approximately 50 cm2. Approximately 60% is lined by stratified squamous epithelium, and most of the remainder is covered by ciliated columnar epithelium (4). Squamous epithelium lines the inferior half of the anterior and posterior walls; it also lines the anterior half of the lateral walls. Ciliated epithelium is present around the nasal choanae and over the roof of the posterior wall. The remainder of the nasopharynx, including the posterior lateral walls and the middle third of the posterior wall, has alternating islands of squamous and ciliated epithelium. There also may be focal areas of “transitional,” or intermediate, epithelium. The latter term is preferred because, although the epithelium resembles urothelium on light microscopy, it lacks the specialized ultrastructural and immunohistochemical features of urinary tract mucosa. Intermediate epithelial cells are less polyhedral than squamous cells, yet they are rounder than columnar ciliated cells and their nonciliated precursors. Intermediate epithelium tends to be concentrated as a wavy ring at the junction of the nasopharynx and oropharynx. Oncocytic metaplasia of the seromucinous glands of the nasopharynx is occasionally an incidental finding in nasopharyngeal biopsies. A few cases have resulted in clinically visible masses, and they may produce symptoms by obstructing the eustachian tube (5).
INFLAMMATORY/NONNEOPLASTIC LESIONS
INFECTIONS
Tissue from patients with common viral and bacterial infections of the upper airway is not seen by the surgical pathologist. However, biopsies of tissue changes caused by infectious diseases that are destructive, involve large areas of mucosa, or result in a mass may be performed.
FUNGI
A variety of fungi affect the nose and sinuses, which may constitute the sole site of infection. Mucormycosis (phycomycosis) is a life-threatening opportunistic infection by organisms of the order Mucorales. Broad nonseptate hyphae, readily seen on hematoxylin and eosin (H&E)-stained sections, spread along nerves, across tissue planes, and into blood vessels. Inflammation ranges from negligible to large numbers of neutrophils and histiocytes within granulation tissue.
Aspergillus organisms are septate hyphae that branch at 45 degrees. Fungus balls can occur in the antrum of immunocompetent people and can elicit a minimal inflammatory reaction of granulation tissue with microabscesses or multinucleated giant cells. Invasive aspergillosis occurs in immunosuppressed and immunocompetent persons (6). Extension into the retro-orbital region, cranial vault, or parapharyngeal space can be fatal (7). Putative allergic reactions to Aspergillus species (not culture proven) have been characterized by pathologic evidence of “allergic mucin” (8,9). The mucin is often so tenacious that it requires curettage for adequate removal, and recurrent disease is common (10). Bone erosion may be present on radiography, but it is caused by pressure remodeling rather than destruction by fungal invasion (10). The mucin contains eosinophils, Charcot-Leyden crystals, and hyphae. The mucin and infiltrate are often laminated. Culture-proven infection by Curvularia lunata and the dematiaceous fungi Drechslera, Bipolaris, and Exserohilum has also resulted in an inflammatory response with the appearance of allergic mucin (11,12). The dematiaceous fungi may be identified by their positive staining with a Fontana-Masson melanin stain (12).
Cryptococcosis (13) and actinomycosis (14) are extremely unusual infections of the nose. Other invasive fungal infections may be caused by Zygomycetes (15), Pseudallescheria boydii (16), Paecilomyces species (17), Alternaria species (18), Cladosporium trichoides (19), and Fusarium (20). Fungi causing noninvasive sinusitis include Pseudallescheria boydii, Sporothrix schenckii, Penicillium meliniiu, Stemphylium mucosporideum, Candida species (21), and Chrysosporium pruinosum (22). Organisms are usually visible on H&E-stained sections, but they may be focal and sparse, requiring methenamine silver to facilitate diagnosis. Inflammatory reactions are purulent, granulomatous, or mixed. The inflammation serves only to alert one to the possibility of an infectious process. It has no diagnostic specificity.
TUBERCULOSIS
Tuberculosis of the sinonasal mucosa is an uncommon manifestation of hematogenous spread; even more rarely, it develops primarily in the nose (23). The septum and inferior turbinate are preferentially involved by either an ulcerated or polypoid lesion. Septal perforation can occur. On microscopic examination, the granulomas are usually poorly formed and almost never necrotic. Sometimes, epithelioid histiocytes intermixed with lymphocytes and plasma cells are the only inflammatory response. It is uncommon to identify organisms in tissue sections, and the diagnosis usually must be established by culture.
LEPROSY
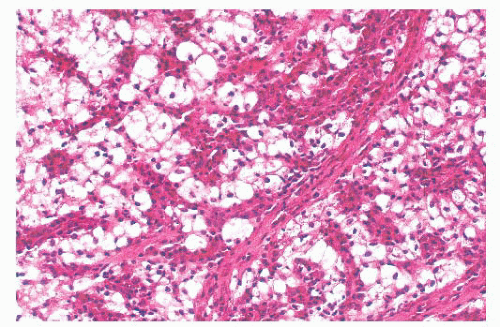
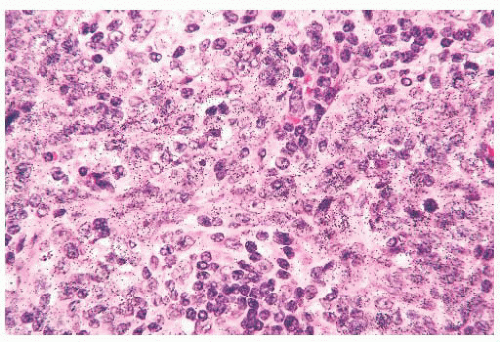
Leprosy affects the nasal mucosa in 95% of patients and sometimes is the initial manifestation of disease (24). As with some other granulomatous inflammations, the inferior turbinate and the septum are the favored sites. In the early stage, there may be only a small number of histiocytes and lymphocytes, with a predominance of plasma cells (Figs. 21.3 and 21.4). Later, the histiocytes form broad sheets containing many foam cells (25). The tuberculoid form of leprosy, with well-formed granulomas, is uncommon in the nose.
RHINOSPORIDIOSIS
RHINOSCLEROMA
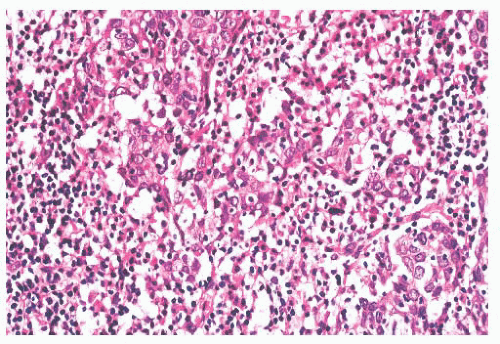
Rhinoscleroma may be confused with leprosy because rhinoscleroma is also characterized by foamy histiocytes (Mikulicz cells) intermixed with plasma cells (Fig. 21.6) (28). The organisms of
leprosy are acid-fast (Fig. 21.4), whereas rhinoscleroma is caused by Gram-negative rods (Klebsiella rhinoscleroma) (Fig. 21.7).
leprosy are acid-fast (Fig. 21.4), whereas rhinoscleroma is caused by Gram-negative rods (Klebsiella rhinoscleroma) (Fig. 21.7).
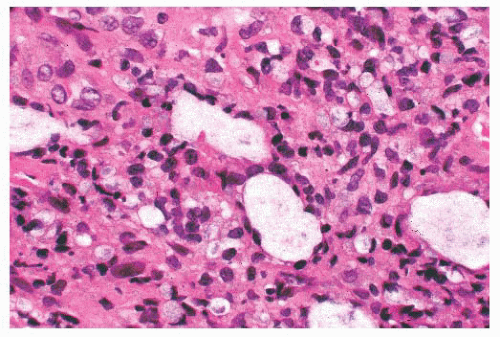 FIGURE 21.3 Nasal leprosy consists of large, foamy histiocytes, without visible nuclei in this microscopic field, in association with a mixed inflammatory infiltrate. |
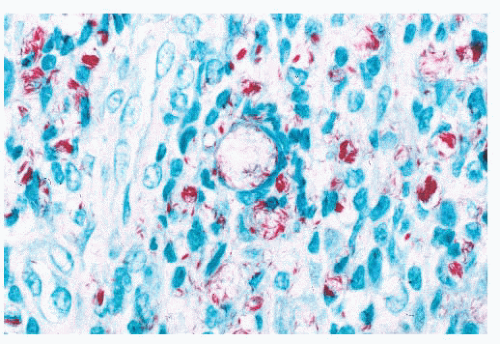 FIGURE 21.4 Acid-fast stain from the case seen in Figure 21.3 shows numerous acid-fast organisms, some forming aggregates. |
MIDFACIAL NECROTIZING LESION (“LETHAL MIDLINE GRANULOMA”)
Midfacial necrotizing lesion (“lethal midline granuloma”) is a clinical designation for one or more infiltrative, often destructive, mucosal lesions of the upper aerodigestive tract (29). This is not a pathologic diagnosis because many diseases, including infections and neoplasms, have clinically similar initial symptoms (30). The three pathologic entities that cause the most diagnostic difficulty are Wegener granulomatosis, natural killer (NK)/T-cell lymphoma, and so-called idiopathic midline destructive disease. These conditions must be sharply separated because of markedly different prognoses and therapies. All are difficult to diagnose on punch biopsies because the only tissue obtained is often secondarily inflamed, ulcerated mucosa. Deep incisional biopsies are usually necessary to obtain sufficient tissue, given the spotty nature of diagnostic areas in
Wegener granulomatosis and NK/T-cell lymphoma. Similarly, the diagnosis of idiopathic midline destructive disease requires generous sampling because it is a diagnosis of exclusion.
Wegener granulomatosis and NK/T-cell lymphoma. Similarly, the diagnosis of idiopathic midline destructive disease requires generous sampling because it is a diagnosis of exclusion.
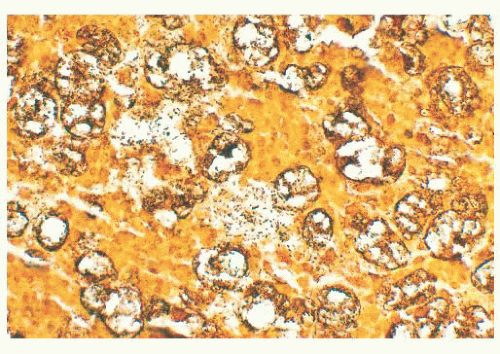 FIGURE 21.7 Large numbers of rod-shaped bacillary organisms are seen in this Steiner stain from the lesion depicted in Figure 21.6. |
Clinical Features
The clinical signs of Wegener granulomatosis, NK/T-cell lymphoma, and idiopathic midline destructive disease share similarities. Patients most often complain of rhinorrhea and sinus pain. The mucosa of the nose, paranasal sinus, oral cavity, and nasopharynx is thickened in variable combinations. Lesions of the nasopharynx often result in otitis media or mastoiditis as a result of eustachian tube involvement. Regardless of the site, the affected mucosa is focally ulcerated. Of the three, Wegener granulomatosis is the least destructive of underlying cartilage or bone in the early stages of disease. On the other hand, patients with NK/T-cell lymphoma and idiopathic midline destructive disease often have a perforated nasal septum, perforated palate, erosion of the bone of the antrum, or ulceration of the skin overlying the nose or antrum (31).
Morphologic Characteristics
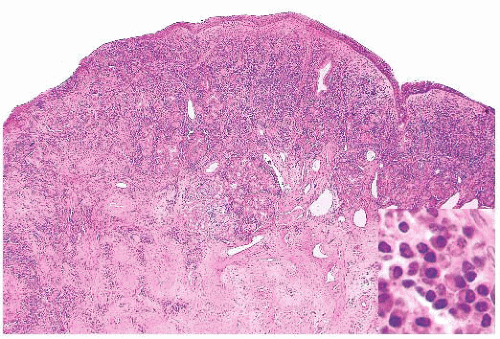
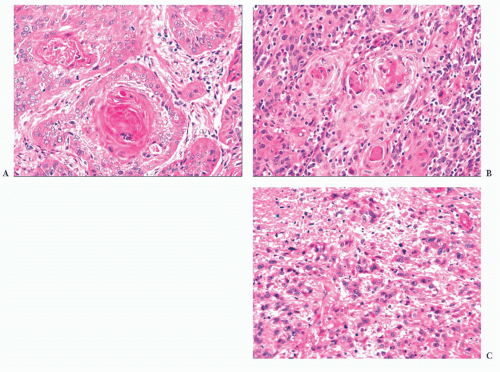
Wegener granulomatosis is characterized by necrotizing vasculitis involving arterioles, small arteries, and veins. The affected vessels are often situated in a broad sheet of extravascular inflammatory cells that makes their detection difficult. An elastic stain is sometimes useful, especially when the vessel lumen has been obliterated by inflammatory cells and thrombus. All stages of vasculitis are present, ranging from acute to healed. The acute vasculitis is characterized by a predominantly neutrophilic infiltrate of the vessel wall, accompanied by patchy fibrinoid necrosis. The inflammation and necrosis can involve small sectors or the entire circumference. Vessels with healed vasculitis have narrowed or obliterated lumina with concentric rims of perivascular collagen (Fig. 21.8). Variable numbers of lymphocytes, plasma cells, or histiocytes are in the wall and surrounding stroma.
Giant cells unassociated with granulomas are usually evident in Wegener granulomatosis, but they may be sparse. Granulomas are rarely well formed, and they most often consist of ill-defined aggregates of mononuclear or multinucleated histiocytes associated with nuclear debris. The giant cells or granulomas may be far from vessels, adjacent to a vessel with vasculitis, or within the vessel wall. Rarely, a granuloma may be centered around fragmented elastic tissue representing the remnants of a totally destroyed vessel.
Patchy foci of necrotic connective tissue and inflammatory cells are invariably present, given adequate tissue sampling. The necrosis is of the coagulative type and may contain degenerating neutrophils (Fig. 21.9). The necrotic areas can have a rim of giant cells with epithelioid or palisaded, spindle-shaped mononuclear histiocytes. The necrotic foci may have a geographic, “punched out” appearance or may be serpiginous. Some researchers consider this type of necrosis and histiocytic response sufficient for the diagnosis, even in the absence of vasculitis (32). In addition to the neutrophils and histiocytes, the extravascular tissue has lymphocytes, plasma cells, and eosinophils. Eosinophils are numerous in a minority of cases and are almost totally absent in others.
Biopsies from the head and neck region, usually the nasal cavity, have been considered to be fully diagnostic in 20% to 41% of patients with Wegener granulomatosis (33,34). Colby et al. (35) noted that biopsies were diagnostic or suggestive of Wegener granulomatosis in approximately 70% of cases. Devaney et al. (33) have proposed the following criteria for the diagnosis of Wegener granulomatosis in head and neck biopsies: (a) The finding of all three microscopic features (granulomatous inflammation, necrosis, and vasculitis) is considered diagnostic if the patient has additional clinical features of involvement of the lung, kidney, or both. (b) If two of the three microscopic features are present, the biopsy is considered diagnostic only if both the lungs and kidneys show clinical signs of disease; if only one additional site is involved, the biopsy is termed “probable.” (c) When only one of the three microscopic features is present, the biopsy is considered suggestive if there is pulmonary and renal involvement or suspicious if only one of these sites is affected. (d) If none of the three microscopic features is present, the biopsy is considered nonspecific, even when there is clinical evidence of pulmonary and renal disease (33).
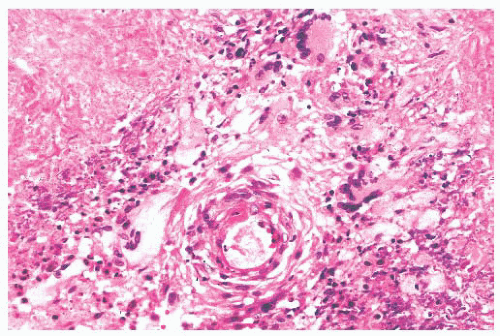 FIGURE 21.9 Wegener granulomatosis has geographic areas of necrosis that may be rimmed in part by multinucleated giant cells. An inflamed vessel is present. |
If necrotizing vasculitis is the only change, one must be careful to distinguish it from secondary inflammation of the vessel wall resulting from some other cause. Similarly, transmigration of neutrophils can mimic vasculitis. In neither instance, however, is fibrinoid necrosis seen. When there is necrosis of the extravascular tissues, with or without giant cells or granulomas, infection must be considered. Stains for acid-fast organisms and fungi are warranted, accompanied by cultures.
Approximately two-thirds of patients with Churg-Strauss syndrome have nasal polyps or mucosal crusting. At the microscopic level, the tissue has discrete necrotizing granulomas with numerous eosinophils that may form microabscesses. In one series, angiitis was absent in the sinonasal lesions (36). Because Wegener granulomatosis sometimes has large numbers of eosinophils and minimal angiitis, the distinction from Churg-Strauss syndrome may rest mainly on clinical grounds.
Another entity that can be confused with Wegener granulomatosis is NK/T-cell lymphoma because the latter often has a considerable inflammatory component. In addition, the atypical lymphocytes tend to be angiocentric, infiltrate the vessel wall, and superficially mimic vasculitis (37). The majority of the cells in the vessel wall, however, are atypical lymphocytes, and the vessel is not necrotic. NK/T-cell lymphoma of the upper respiratory tract has been referred to by a variety of terms, including midline malignant reticulosis and polymorphic reticulosis. These lesions are described in detail in the later section on NK/T-cell lymphoma. The pulmonary lesion of so-called lymphomatoid granulomatosis (38,39) can secondarily involve the upper respiratory tract, producing lesions that are similar to primary NK/T-cell lymphomas in many morphologic features, including a distinctly angiocentric growth pattern. Although previously thought to be synonymous with these lesions, lymphomatoid granulomatosis is now believed to represent a distinctly different process, a T-cell-rich B-cell lymphoma. Both processes share a strong association with Epstein-Barr virus (EBV) infection, however.
Idiopathic midline destructive disease (IMDD) is characterized by normal-appearing acute and chronic inflammatory cells with variable amounts of necrosis. Atypical lymphocytes are absent, thus eliminating the possibility of NK/T-cell lymphoma. The inflammatory cells occasionally infiltrate the walls of small vessels, but fibrinoid necrosis is absent, which distinguishes it from Wegener granulomatosis. Eosinophils are inconspicuous. Multinucleated giant cells and granulomas have been described in a few cases of IMDD. In instances where the pathologic findings are ambiguous, the clinical differences from Wegener granulomatosis, emphasized by Tsoskos et al. (40), may be influential in reaching the correct clinicopathologic diagnosis. Cocaine users can have a midfacial destructive syndrome that closely mimics IMDD (Fig. 21.10). Although some have suggested a less fulminant course in cocaine abusers (41), it seems likely that many examples of IMDD represent unrecognized cases of cocaine abuse. Indeed, IMDD most likely represents a heterogeneous group of lesions rather than a distinct entity.
SARCOIDOSIS
Approximately 5% of patients with sarcoidosis have upper airway disease, and the nose is rarely the first site of involvement (42,43). As with tuberculosis, the nasal septum and inferior turbinate are the preferred sites. The nonnecrotic granulomas of sarcoid and tuberculosis can be identical at the light microscopic level (Fig. 21.11).
NONINFECTIOUS GRANULOMATOUS REACTIONS
Steroid injections of the nasal mucosa can produce granulomas with palisades of histiocytes surrounding an amorphous area. The amorphous zone probably is the residual injected substance (44). Nonnecrotic granulomas are occasionally found in otherwise unremarkable inflammatory nasal polyps and sometimes in relation to small nasal ulcers. The granulomas, which are of unknown origin, are usually just beneath the epithelium, may resolve spontaneously, and do not recur after excision (45). Nasal granulomas have been reported in a patient with Crohn disease (46). Cholesterol granulomas of the sinuses consist of (a) a foreign body giant cell reaction around empty, needle-shaped spaces and (b) a chronic inflammatory reaction with hemosiderin. As with cholesterol granulomas of the middle ear, hemorrhage is the presumed pathogenesis, although a specific cause is rarely identified (47). Cholesteatoma, a foreign body giant cell response to keratin, has been described in the maxillary antrum (45).
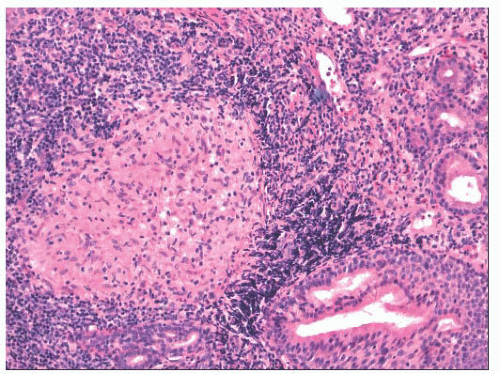 FIGURE 21.11 Nasal sarcoidosis. A well-formed noncaseating granuloma is present along with dense chronic inflammation, adjacent to seromucinous glands of the nasal mucosa. |
MYOSPHERULOSIS
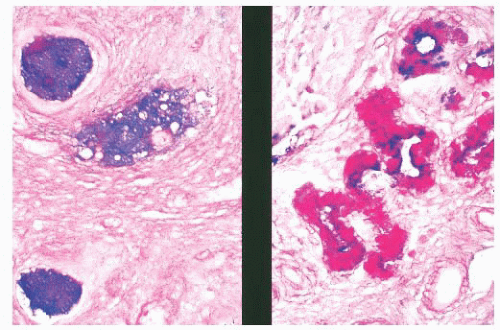
Myospherulosis is an inflammatory and fibrous reaction that surrounds encysted, degenerating erythrocytes (Fig. 21.12) (48,49). This iatrogenic condition follows surgical procedures when an oilbased hemostatic packing has been used. Symptomatic reaction to the oil may necessitate reoperation (50). The encysted erythrocytes mimic Prototheca but do not stain with methenamine silver.
MUCOCELE
Mucoceles of the paranasal sinuses are generally considered to arise from obstruction of the ostium as a result of inflammation, trauma, osteoma, or, occasionally, a neoplasm (51). Two-thirds of mucoceles are in the frontal sinus, and most of the rest are in the anterior ethmoid sinus. The resected mucosa is either normal or compressed ciliated epithelium that sometimes has squamous metaplasia. Mucus may be extravasated in the lamina propria, where it can be phagocytosed by histiocytes (mucophages).
NONSECRETORY CYST
Rarely, cysts of the antrum will be lined by fibroblasts. They have been termed nonsecretory cysts and are thought to originate from edema beneath the mucous membrane stemming from infection or allergy. The accumulation of fluid forms multiple spaces that eventually coalesce into a single cyst lined by flattened fibroblasts rather than epithelium (52).
NECROTIZING SIALOMETAPLASIA
Necrosis of seromucinous glands with secondary squamous metaplasia after surgery on the nose or sinuses produces the microscopic image of necrotizing sialometaplasia (53). The benign, although focally atypical, cytologic appearance of the cells and, more importantly, maintenance of the acinar architecture distinguish necrotizing sialometaplasia from either squamous carcinoma or mucoepidermoid carcinoma. This entity is illustrated and discussed in detail in Chapter 20.
NASAL POLYPS
Polyps of the nasal cavity and paranasal sinuses are nonneoplastic stromal and epithelial proliferations of uncertain pathogenesis. Although all nasal and paranasal polyps share morphologic similarities, there are three clinically—and, to a lesser extent, microscopically—distinctive subtypes.
INFLAMMATORY POLYPS
Clinical Features
Inflammatory polyps are by far the most common. They are almost invariably multiple and typically bilateral, and they involve both the nasal cavity and the paranasal sinuses. To find them in patients less than 20 years of age is distinctly uncommon; most patients are more than 30 years old. An association with asthma or chronic rhinitis, often with negative skin allergen tests, is well established. Approximately 14% of patients with nasal polyps manifest aspirin intolerance, usually in the form of a bronchospastic, asthmatic reaction (54). The presumed mechanism of bronchospasm is a defect in prostaglandin metabolism.
Morphologic Characteristics
On gross inspection, these polyps have a translucent, moist, or edematous cut surface. Most have broad bases of attachment. Areas of opacity should be sectioned to exclude other processes, such as inverted papilloma. At the microscopic level, nasal polyps are localized outgrowths of lamina propria resulting from the accumulation of edema-like fluid with varying degrees of fibroblastic proliferation and inflammation (Fig. 21.13). Mucous glands are embedded in the stroma, particularly in the distal portion of the polyp. The glands vary greatly in number but are typically less numerous than those in normal mucosa. They have a distinctly tubular configuration, connect to the surface of the polyp, and probably arise secondarily after the polyp has begun to form (55). Polyps with prominent glandular elements should not be mistaken for low-grade adenocarcinoma (56) or polypoid mixed tumors of the salivary type (57). The basement membrane underlying the surface mucosa is markedly thickened in most, but not all, inflammatory polyps. The amount and composition of the inflammatory component are highly variable. There may be a predominance of neutrophilic, eosinophilic, or
lymphoplasmacytic elements. Attempts to differentiate “allergic” nasal polyps from inflammatory polyps on the basis of tissue eosinophilia have given inconsistent results.
lymphoplasmacytic elements. Attempts to differentiate “allergic” nasal polyps from inflammatory polyps on the basis of tissue eosinophilia have given inconsistent results.
NASAL POLYPS IN CYSTIC FIBROSIS
Clinical Features
Inflammatory nasal polyps are rare in childhood, except in children with cystic fibrosis. In the latter population, they are seen in up to 20% of children (58). The majority of polyps occur in patients with documented disease, but there are examples of nasal polyps occurring up to 12 years before the diagnosis of cystic fibrosis (59). For this reason, it is important to suspect this condition in any child with an inflammatory-type nasal polyp. Rarely, nasal polyps may be the initial manifestation in adults with cystic fibrosis.
Morphologic Characteristics
Nasal polyps in cystic fibrosis closely resemble inflammatory polyps, but there are minor differences that, when taken in aggregate, usually allow the pathologist to make the distinction (60). Polyps in cystic fibrosis lack basement membrane thickening and submucosal hyalinization, and they usually contain few stromal eosinophils. More important, the mucous glands, cysts, and blanket contain predominantly acid mucin, manifested as a blue or purple-blue coloration with combined Alcian blue/periodic acid-Schiff stain (60). In contrast, the neutral mucin in inflammatory polyps stains red or purple-red (Fig. 21.14). Goblet cells in both conditions contain acid mucin.
ANTROCHOANAL POLYPS
Clinical Features
Antrochoanal polyps originate from a wall of the maxillary antrum. They extend via a long stalk through a large primary or accessory maxillary ostium into the nasal cavity. With continued growth, they often pass through the choana and into the nasopharynx (61,62,63,64 and 65). Large antrochoanal polyps may even be visible in the back of the oropharynx. In contrast to typical nasal polyps, antrochoanal polyps are less common (4% to 6% of all nasal polyps) and frequently occur in childhood (61,62 and 63). Approximately 90% are solitary (63).
Morphologic Characteristics
Resected antrochoanal polyps have a long, narrow stalk, and the body of the polyp is usually firm and fibrous (Fig. 21.15). On microscopic examination, the surface mucosa is thin and usually lacks the thickened basement membrane of inflammatory polyps. The stroma is variable, but frequently, it is less edematous and more fibrotic, and it contains fewer glands than that of typical nasal polyps. Large vascular spaces may be present. Stromal inflammation is patchy, and only approximately 20% of antrochoanal polyps contain prominent eosinophils (61).
STROMAL ATYPIA IN POLYPS
Most, if not all, polyps of the nasal cavity and paranasal sinuses have scattered, mildly atypical stromal cells (66,67). Occasionally, such polyps contain sufficient numbers of pleomorphic or overtly bizarre stromal cells to lead to its confusion with a sarcoma (66,67 and 68). Atypical cells are most numerous in polyps from younger individuals (66) or in polyps with a prominent fibrous stroma (67). Antrochoanal polyps may develop marked degrees of this change, perhaps because of their frequent occurrence in childhood and typically fibrous stroma (69). Similar, overtly bizarre pseudomalignant changes have been described in granulation tissue removed from the paranasal sinuses after radiation therapy (71).
The atypical stromal cells tend to be concentrated in the submucosal region or near small vascular channels. They are characteristically spindled and have variably enlarged, angulated, dark nuclei (Fig. 21.16). Nuclear chromatin may have a bubbly appearance or may be densely hyperchromatic. A few cells may have small, distinct nucleoli. Eosinophilic cytoplasm is usually abundant and parallels nuclear size, such that the nucleus-tocytoplasm ratio tends to remain constant. Despite their individually alarming appearance, the cells are haphazardly arranged
in otherwise typical, fibrous to edematous stroma. There is no increased cellularity or neovascularity, and mitotic figures are extremely rare or absent. Confusion with sarcoma, particularly rhabdomyosarcoma, is avoided if these features are noted and if the low-power pattern of a polyp with stromal glands is observed. Immunocytochemical studies of the atypical cells have documented fibrohistiocytic features (68).
in otherwise typical, fibrous to edematous stroma. There is no increased cellularity or neovascularity, and mitotic figures are extremely rare or absent. Confusion with sarcoma, particularly rhabdomyosarcoma, is avoided if these features are noted and if the low-power pattern of a polyp with stromal glands is observed. Immunocytochemical studies of the atypical cells have documented fibrohistiocytic features (68).
CHEMOTHERAPY-INDUCED ATYPIA
Patients undergoing aggressive chemotherapy that includes alkylating agents (busulfan, cyclophosphamide, etc.), usually for the treatment of hematopoietic malignancies, have been noted to develop at least transient bizarre epithelial atypia in their nasal mucosa (70). Changes include striking nuclear enlargement and pleomorphism (Fig. 21.17). Biopsies from this region are often obtained during immunocompromise to assess for opportunistic fungal or other infectious processes. The atypia, particularly when it occurs in an area of squamous metaplasia, may be misdiagnosed as dysplasia. It may also be confused with cytomegalovirus or herpesvirus cytopathic effect. It is unclear how long these changes persist after cessation of treatment.
BENIGN NEOPLASMS AND NEOPLASM-LIKE PROCESSES
SQUAMOUS AND SCHNEIDERIAN PAPILLOMAS
Papillomas of the nose and paranasal sinuses include lesions lined with mature squamous epithelium and histologically diverse schneiderian papillomas. Papillomas lined with mature squamous epithelium usually develop in the vestibule of the nose, where they originate from skin. Rarely, the nasopharynx is involved in patients with extensive juvenile papillomatosis (72).
The schneiderian papillomas have many names, reflecting their different microscopic features. The major types are fungiform papilloma, inverted papilloma, and oncocytic schneiderian papilloma. The latter historically has been referred to as cylindrical cell papilloma, whereas fungiform papilloma and inverted papilloma have often been called transitional cell papilloma or, simply, papillomatosis. The role of human papillomavirus (HPV) in the development of schneiderian papillomas has been the subject of numerous studies. In situ hybridization and polymerase chain reaction (PCR) studies have shown papillomavirus DNA in almost all fungiform papillomas and in a minority of inverted papillomas (73,74,75 and 76). Cylindrical cell papillomas have been negative for HPV DNA (73,74,77). EBV has not been detected in schneiderian papillomas (74).
Clinical Features
The schneiderian papillomas develop in patients who are mainly between 30 and 50 years of age, with men being affected twice as often as women. Unilateral nasal obstruction is the most common complaint, but epistaxis, facial pain, purulent discharge, and proptosis may also occur. The latter symptom has been reported only with inverted papilloma and reflects its ability to erode bone by pressure. Although the papillomas are often multifocal, bilaterality is rare. All three types have a recurrence rate of 50% to 70% (usually within 1 to 2 years) if treated only by local excision. The recurrences are usually microscopically identical to the original lesion unless carcinoma has supervened. However, some authors report greater atypia and a higher rate of mitoses in recurrences (78). The recurrence rate can be reduced to approximately 5% for papillomas involving the lateral nose and antrum by performing a medial maxillectomy (79). The rate of recurrence has not been consistently related to the histologic features.
Morphologic Characteristics
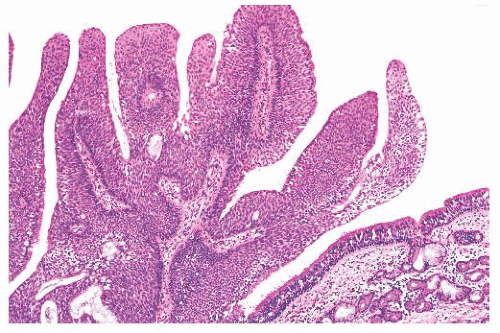
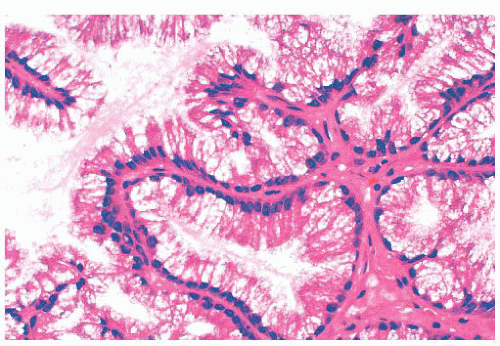
On gross inspection, all three types of schneiderian papillomas are soft to moderately firm, with a granular or finely clefted surface (Fig. 21.18). The cell types in fungiform and inverted papilloma are the same, but the two differ architecturally, in their site of origin and in their association with carcinoma. Fungiform papilloma arises almost exclusively on the nasal septum, whereas the inverted form predominantly affects the lateral wall of the nose and/or the paranasal sinuses. Fungiform
papilloma is not associated with carcinoma, whereas inverted papilloma is (vide infra) (80,81 and 82).
papilloma is not associated with carcinoma, whereas inverted papilloma is (vide infra) (80,81 and 82).
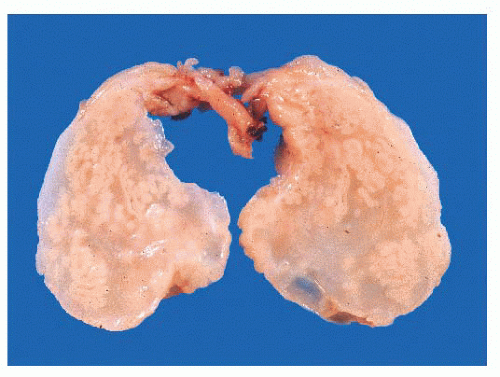 FIGURE 21.18 Inverted papilloma. The stroma is edematous much like an inflammatory polyp, but the epithelial invaginations are clearly evident. |
There is some architectural overlap between fungiform and inverted papilloma. Generally, fungiform papilloma has connective tissue stalks that form an exophytic architecture (Fig. 21.19). By contrast, inverted papilloma has invaginations of the surface epithelium into the underlying stroma (Fig. 21.20). Nonetheless, some inverted papillomas will have an exophytic surface in addition to the invaginations, and fungiform papilloma can have sporadic invaginations. The epithelium of fungiform and inverted papilloma includes squamous, ciliated columnar, intermediate, and mucus-secreting cells.
Nonkeratinizing squamous epithelium is most common, followed by intermediate epithelium (Fig. 21.21). These epithelial types range from 5 to 30 cells in thickness. Ciliated epithelium has the appearance of normal respiratory mucosa, although ciliated cells occasionally form a single layer on top of intermediate or squamous cells. The mucous cells have distended cytoplasm with an eccentric nucleus. They usually are seen individually but occasionally form small clusters. All epithelial types may be present in a single papilloma. Mitotic figures are usually few in number and are located in the basal or parabasal region, but they may be more numerous and can be found in the upper half of the epithelium as well. Atypical forms are absent.
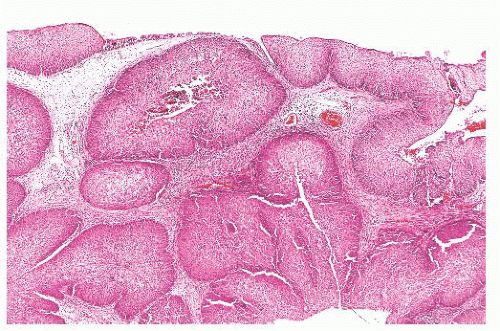 FIGURE 21.20 This inverted papilloma consists of deeply invaginated nests of benign, predominantly squamous epithelium. |
Nuclear pleomorphism is prominent in approximately 10% of inverted papillomas, mainly as a few isolated cells at any depth within the epithelium. Sometimes, atypical nuclei form larger aggregates in the inner third of the mucosa. Neutrophils can be scattered throughout the epithelial layer as single cells or microabscesses. This inflammation imparts a disquieting histologic appearance to the lesion, especially when it is accompanied by nuclear abnormalities. The stroma is variably fibrous, edematous, or vascular. Inflammatory cells can be absent or abundant. Plasma cells, lymphocytes, and neutrophils predominate, but eosinophils are sometimes numerous. Unlike inflammatory polyps, seromucinous glands are not present, except at the base of the lesion, where they arise from the mucosa.
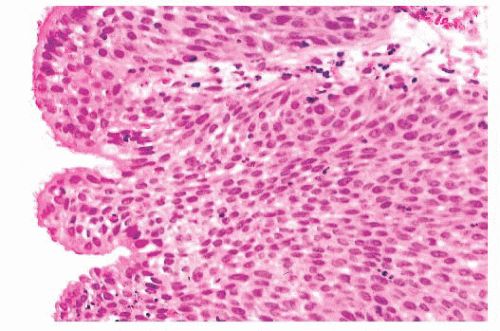 FIGURE 21.21 Inverted papilloma and fungiform papilloma are often lined by intermediate or ciliated epithelium. Mucous cells may be interspersed. |
The differential diagnosis of inverted and fungiform papilloma includes nonkeratinizing squamous carcinoma when it has either a papillary architecture or narrow, serpiginous ribbons of
invading epithelium. On cytologic examination, however, papillary carcinoma has widespread, severe nuclear abnormalities (Figs. 21.22 and 21.23), and often the cells have a partially clear cytoplasm. Papillary squamous carcinoma frequently has scattered small, round nests of mature squamous epithelium.
invading epithelium. On cytologic examination, however, papillary carcinoma has widespread, severe nuclear abnormalities (Figs. 21.22 and 21.23), and often the cells have a partially clear cytoplasm. Papillary squamous carcinoma frequently has scattered small, round nests of mature squamous epithelium.
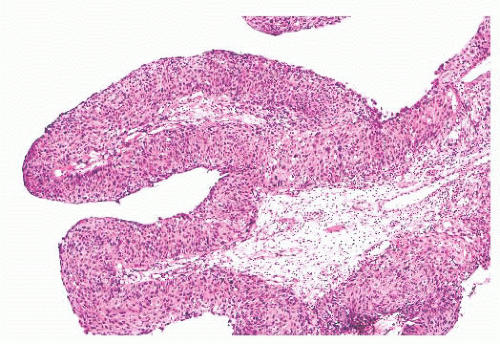 FIGURE 21.22 This nasal papillary carcinoma has well-developed fibrovascular cores and superficially resembles a fungiform papilloma. |
Oncocytic schneiderian papilloma has areas of intermediate cell proliferation identical to inverted papilloma, and the lowpower architecture of these two papillomas is indistinguishable. In contrast to inverted papilloma, however, the majority of the cells in oncocytic papilloma have a finely granular eosinophilic cytoplasm and less sharply defined cell borders (Figs. 21.24 and 21.25). The outermost layer is often ciliated, and the cells rarely form a layer more than six to eight cells thick. Oncocytic papilloma frequently contains numerous cells with sharply delimited, inspissated mucin droplets (82).
The differential diagnosis of oncocytic papilloma includes inflammatory polyp. When there are many neutrophils in the epithelium and stroma of oncocytic papilloma, the resemblance to an inflammatory polyp is enhanced. Inflammatory polyps, however, do not have the oncocytic epithelial cells or mucous inclusions. The numerous vacuoles containing inspissated mucus superficially resemble rhinosporidiosis. The organisms of rhinosporidiosis, however, involve both the epithelium and underlying stroma. Moreover, they are not accompanied by oncocytic epithelium. When the mucosa of oncocytic papilloma is cut tangentially, the numerous small lumina may mimic adenocarcinoma. However, adenocarcinomas of the nose or paranasal sinuses rarely have eosinophilic cytoplasm. The lack of nuclear atypia in oncocytic papilloma further distinguishes it from carcinoma.
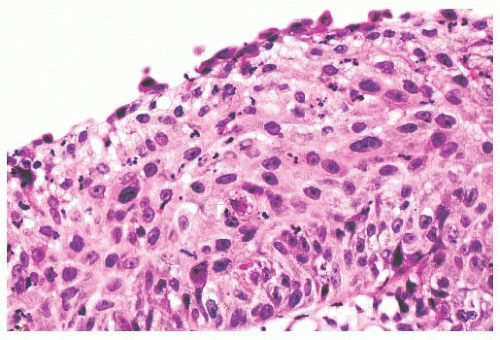 FIGURE 21.23 A higher power view of the epithelium seen in Figure 21.18 shows mildly pleomorphic, hyperchromatic, and somewhat disorganized squamous cells. A suprabasal mitotic figure is present. |
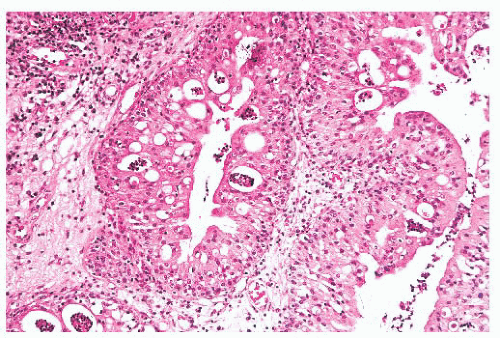 FIGURE 21.24 Oncocytic schneiderian papilloma has irregularly distributed strips of eosinophilic cells with interspersed mucous cells and acute inflammation. |
For practical purposes, inverted and oncocytic papillomas are not metastasizing lesions in the absence of carcinoma. Two patients with inverted papilloma have had lesions in the neck (83,84). It is debatable whether these represent true metastases, implants in lymph nodes, or papillary transformation of a branchial cleft epithelial remnant. Rarely, inverted papilloma extends up the eustachian tube and involves the middle ear and mastoid (85).
The association of carcinoma with inverted and oncocytic papillomas is indisputable. Invasive squamous cell carcinoma occurs in approximately 6% to 14% of patients with these papillomas (86,87 and 88). In our experience, as well as that of others, the oncocytic variants seem most prone to undergo malignant transformation (86,88). Patients with carcinoma have a mean
age 9 to 13 years older than the mean age of all patients with inverted papilloma (89). The carcinoma may be within the papilloma at the time the patient is first seen or may arise after an interval that may or may not have included recurrent papillomas. Metachronous carcinoma may or may not be associated with a papilloma. There are no criteria to predict which papillomas will be followed by carcinoma. The rate at which papillomas recur and the length of time between recurrences do not correlate with the risk of subsequent carcinoma. The carcinomas are mostly of the squamous cell type (90), but spindle cell (91), clear cell (92), high-grade mucoepidermoid (82,92), and sinonasal undifferentiated carcinomas (SNUCs) (93) have also been described. All tissue removed from a patient with inverted or oncocytic papilloma should be submitted for microscopic examination so as not to overlook small foci of carcinoma.
age 9 to 13 years older than the mean age of all patients with inverted papilloma (89). The carcinoma may be within the papilloma at the time the patient is first seen or may arise after an interval that may or may not have included recurrent papillomas. Metachronous carcinoma may or may not be associated with a papilloma. There are no criteria to predict which papillomas will be followed by carcinoma. The rate at which papillomas recur and the length of time between recurrences do not correlate with the risk of subsequent carcinoma. The carcinomas are mostly of the squamous cell type (90), but spindle cell (91), clear cell (92), high-grade mucoepidermoid (82,92), and sinonasal undifferentiated carcinomas (SNUCs) (93) have also been described. All tissue removed from a patient with inverted or oncocytic papilloma should be submitted for microscopic examination so as not to overlook small foci of carcinoma.
Respiratory Epithelial Adenomatoid Hamartoma
The term respiratory epithelial adenomatoid hamartoma (REAH) was coined by Wenig and Heffner (94) to refer to a proliferation of glandular spaces lined by ciliated epithelium and, less often, goblet cells. The glands are situated in a stromal background resembling inflammatory polyp, replete with edema and chronic inflammatory cells (Figs. 21.26 and 21.27). The lesion arises most often on the posterior nasal septum, but it may occur in the lateral nasal mucosa, the nasopharynx, or the paranasal sinuses. The patients of Wenig and Heffner (94) were 27 to 82 years of age, with a median age of 58 years. Many had a history of chronic rhinosinusitis. One patient had an inverted papilloma in continuity with the REAH, and one had a solitary fibrous tumor in the contralateral nasal fossa. REAH did not recur during the follow-up time of 4 months to 5 years. The tumors measured as large as 4.9 cm, but the destructive growth of schneiderian papillomas was absent. There have been scattered additional descriptions of this lesion as case reports, but the author’s experience suggests that this entity is relatively common and often interpreted as an inflammatory polyp with prominent epithelium or as a schneiderian papilloma. Although initially considered to be nonneoplastic, one recent study has suggested otherwise (95). As discussed in the following sections, we have seen several examples of low-grade sinonasal adenocarcinomas arising in REAHs (96).
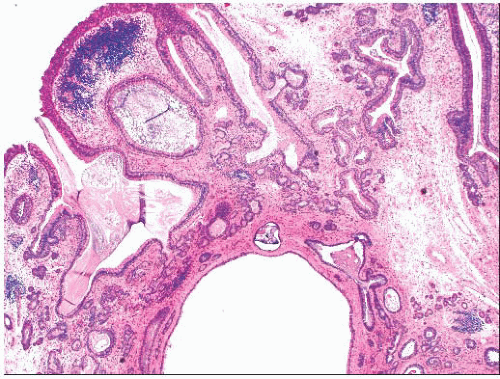 FIGURE 21.26 Respiratory epithelial adenomatoid hamartoma has the low-power appearance of an inflammatory polyp but contains large numbers of irregular glands. |
CHONDROMESENCHYMAL HAMARTOMA
Chondromesenchymal hamartomas almost always arise in children less than 3 months of age (97). Most involve the nasal cavity, but the sinuses may also be affected. Patients usually present with a mass or difficulty breathing. The lesions are well circumscribed and do not recur if completely excised. Histologically, there is a proliferation of various mesenchymal elements, predominantly hyaline cartilage, often in a lobular pattern (Fig. 21.28). The intervening stroma is composed of bland spindled cells and can appear either hypercellular or myxoid. Osteoclast-like giant cells can be present, as can vascular proliferation.
MALIGNANT NEOPLASMS
SQUAMOUS CELL CARCINOMA
Nose and Paranasal Sinuses
Carcinoma of the nose and paranasal sinuses accounts for only approximately 3% of head and neck neoplasms. Approximately 58% develop in the maxillary antrum, 30% develop in the nasal cavity, 10% develop in the ethmoid, and 1% develop in the sphenoid and frontal sinuses (98). Squamous cell carcinoma of the nasal cavity is related to cigarette smoking (99,100) and industrial exposure to nickel ore (101). Job-related exposures to chromium, isopropyl alcohol, and radium also have been associated with sinonasal squamous cell carcinoma (102). Thorotrast has been linked with squamous cell carcinoma of the maxillary antrum (103). Although chronic recurrent sinusitis with secondary squamous metaplasia has been suggested as a factor in the development of sinonasal carcinoma, it is quite possible that, in many such cases, an occult carcinoma is responsible for the recurrent infections, rather than the reverse.
HPV has become well established in the last decade as a cause of an already substantial and increasing number of oropharyngeal squamous carcinomas, particularly of the papillary and nonkeratinizing (basaloid) subtypes and arising from the palatine and lingual tonsils. However, its role in the development of squamous cell carcinomas of the nose and paranasal sinuses is less well studied. DNA from HPV types 16 and 18 has been detected, using the highly sensitive PCR, in one study in 14% of squamous cell carcinomas of the sinonasal region (104). Using this technique, however, apparently normal mucosa in the ear, nose, and throat region also has been noted to contain HPV DNA, raising questions about the significance of this finding. More specific in situ hybridization studies for high-risk HPV DNA have implicated this virus in a minority of sinonasal squamous cell carcinomas, particularly of nonkeratinizing type (105). In this location, as in the oropharynx, strong staining of the neoplastic cells for p16 is a good surrogate for the presence of HPV DNA. The relationship between HPV and schneiderian papillomas has been discussed earlier.
Similarly, EBV has been strongly linked to undifferentiated and nonkeratinizing nasopharyngeal carcinomas, but its association (if any) with sinonasal carcinomas is less clear. The finding of EBV by PCR in conventional squamous cell carcinomas is of questionable significance, given the high prevalence of latent EBV in normal lymphocytes. In our experience, EBV is not associated with keratinizing squamous cell carcinomas anywhere in the head and neck, and an association with mucosal undifferentiated or nonkeratinizing squamous carcinomas outside of the nasopharynx rarely, if ever, occurs.
Clinical Features.
Squamous cell carcinoma of both the nasal cavity and paranasal sinuses shows a male predominance of approximately 2:1 (106). Most patients are in their sixth or seventh decade of life, and cases in patients under 40 years of age are extremely rare. Nasal lesions cause obstruction, rhinorrhea, epistaxis, or pain. An origin in the paranasal sinuses produces symptoms identical to those of chronic sinusitis (98). Regardless of the mode of therapy (surgery, radiation, and/or chemotherapy), recurrences are common; death is usually a result of local spread. The prognosis varies with stage (106).
Nasal cavity carcinoma is associated with a high rate (6% to 28%) of second primary neoplasms (99,106). Other neoplasms may antedate, postdate, or occur concurrently with nasal carcinoma. Approximately 40% of the second primaries involve the head and neck, with the remainder developing at sites such as lung, breast, and gastrointestinal tract (106). In contrast, carcinoma of the maxillary antrum carries a 5% incidence of a second primary carcinoma in the contralateral antrum but no increased rate of occurrence of carcinoma at other sites (107).
Morphologic Characteristics.
The majority of sinonasal squamous cell carcinomas are focally keratinizing, easily diagnosable lesions (Fig. 21.29). Nonkeratinizing carcinomas occasionally occur in this region; rarely, undifferentiated carcinomas (lymphoepithelioma-like) analogous to those of the nasopharynx may develop. Nonkeratinizing carcinomas have been referred to by a variety of terms, including transitional cell carcinoma, intermediate-cell carcinoma, and schneiderian carcinoma. In our experience, these neoplasms are difficult to recognize as a morphologically distinctive group, and we prefer to view them as within the spectrum of squamous cell carcinoma. Nonkeratinizing carcinomas do tend to grow as large cell nests and anastomosing cords of cells, often with only mild cellular pleomorphism. Such carcinomas may be confused, on cursory examination, with fungiform or inverted nasal papillomas because of their architectural similarities. Closer inspection will reveal a degree of mitotic activity and pleomorphism beyond that seen in papillomas. Some larger nests of carcinoma may also have central, comedo-like necrosis.
Unusual variants of squamous cell carcinoma, such as spindle cell carcinoma or sarcomatoid carcinoma, have been described in the sinonasal region (108). These are described and illustrated in the subsequent chapter on laryngeal pathology. If islands of conventional carcinoma are present, the diagnosis is straightforward. Biopsies containing only spindle cells may be difficult or impossible to distinguish from sarcoma or amelanotic melanoma on H&E-stained sections. Immunocytochemistry may demonstrate focal epithelial differentiation in the spindle cell component (108). Although it may be tempting to label high-grade spindle cell lesions in this region lacking evidence of epithelial or specific mesenchymal differentiation as sarcomas, experience indicates that their behavior is that of a high-grade carcinoma. These tumors are discussed in more detail in relation to the larynx and oral cavity.
Verrucous carcinoma of the nasal cavity and paranasal sinuses has been reported (109) but is much less common than its morphologically identical oral and laryngeal counterparts. Manivel et al. (110) reported two unusual sinonasal and nasopharyngeal carcinomas containing areas of endodermal sinus (yolk sac) tumor, replete with α1-antitrypsin and α-fetoprotein expression.
Nasopharynx
In the United States, 78% to 90% of malignant neoplasms arising in the nasopharynx are nonglandular carcinomas (111). In some areas of Asia, where nasopharyngeal carcinomas are much more common, they account for up to 98% of all nasopharyngeal malignancies (111,112). Acquiring an understanding of these tumors was hampered by a lack of uniform nomenclature before 1978, when the World Health Organization published its classification scheme. Under this system, nonglandular nasopharyngeal carcinomas are subtyped as keratinizing squamous cell carcinoma (type 1), nonkeratinizing carcinoma (type 2), and undifferentiated carcinoma (lymphoepithelioma). The morphologic criteria for these groups are discussed later in this chapter.
Clinical Features.
Many nasopharyngeal carcinomas arise near the eustachian tube opening in the fossa of Rosenmüller. Initial complaints are often caused by middle ear obstruction (e.g., otitis, hearing loss) or local invasion (e.g., headache, cranial nerve deficits, epistaxis) (113,114 and 115). Approximately 50% to 80% of patients with undifferentiated carcinoma are initially seen with cervical lymph node metastases from an occult primary (113,114); 25% have bilateral nodal involvement (113,114). Involved lymph nodes are typically posterior to the sternocleidomastoid at the level of the angle of the jaw (114). Examination of the nasopharynx may show entirely normal features, may reveal fullness or surface granularity, or may demonstrate an obvious carcinoma. In patients with a normal-appearing nasopharynx, random biopsies yield diagnostic tissue in 70% of cases; the remainder may require rebiopsy (114).
Squamous cell carcinoma and nonkeratinizing carcinoma are rare in childhood (111,115). In contrast, undifferentiated carcinoma often occurs in children and shows a distinctly bimodal age distribution, with peaks in the second and sixth decades (111,114,115,116,117 and 118). All forms of nasopharyngeal carcinoma have a male predominance of approximately 2.5 to 3:1 (111,114,115). Nasopharyngeal carcinoma is treated exclusively with radiation; survival correlates with the stage of disease and its histologic type. Keratinizing squamous cell carcinomas are the least radiation sensitive and have the poorest prognosis; there were no 5-year survivors reported in one series (113). Nonkeratinizing carcinomas have been reported variously as having a survival rate intermediate between keratinizing and undifferentiated carcinoma (113) or as behaving like undifferentiated carcinoma (111). In several large historical series, patients treated with radiation therapy for undifferentiated carcinoma had the following 5-year survival rates, according to stage: stage I (confined to the nasopharynx), 50% to 60%; stage II (cervical node involvement), 20% to 30%; and stage III (invasion of surrounding structures), 5% to 20% (111,113,114). More recent studies using combined platinum-based chemotherapy and radiation therapy have reported overall 5-year survival rates of 70% to 90% for these patients (119).
In Hong Kong, nasopharyngeal carcinoma accounts for 18% of all malignant neoplasms (120), as opposed to approximately 2% in the United States. Racial susceptibility tends to remain constant in those emigrating to other countries. Chinese patients in Singapore have significantly higher rates of the histocompatibility antigens HLA-A2 and HLA-BW46 (121). In addition, the incidence of nasopharyngeal carcinomas may be lower in patients with blood group A (122). The association between EBV infection and nasopharyngeal carcinoma of the undifferentiated and nonkeratinizing types is well established (111,123,124,125,126,127 and 128). EBV genomic RNA has been repeatedly demonstrated in the neoplastic epithelial cells of
undifferentiated (and some nonkeratinizing) nasopharyngeal carcinomas using an in situ hybridization technique (Fig. 21.30) (111,117,127). The detection of serum antibodies against viral proteins, particularly immunoglobulin A (IgA) and immunoglobulin G (IgG) directed against viral capsid antigen, may be useful in the diagnosis of metastases from occult primary tumors (123,124 and 125). Because of the high frequency of EBVinfected lymphocytes in the general population, PCR studies for EBV are fraught with false-positive results.
undifferentiated (and some nonkeratinizing) nasopharyngeal carcinomas using an in situ hybridization technique (Fig. 21.30) (111,117,127). The detection of serum antibodies against viral proteins, particularly immunoglobulin A (IgA) and immunoglobulin G (IgG) directed against viral capsid antigen, may be useful in the diagnosis of metastases from occult primary tumors (123,124 and 125). Because of the high frequency of EBVinfected lymphocytes in the general population, PCR studies for EBV are fraught with false-positive results.
Morphologic Characteristics.
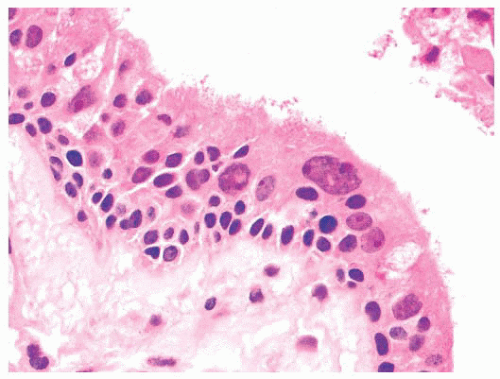
Keratinizing squamous cell carcinoma of the nasopharynx is defined as a tumor having squamous differentiation in the form of intercellular bridges or keratinization over most of its extent (111). These tumors seldom pose diagnostic difficulties except for rare “adenoid” or acantholytic forms that may be confused with adenocarcinoma (126) or even angiosarcoma. Nonkeratinizing nasopharyngeal carcinoma has cells at differing levels of maturation but lacking light microscopic evidence of overt cytoplasmic keratinization. Nuclei vary in size, shape, and chromatin distribution. Tumor cells have well-defined cell margins that interdigitate in a “pavement stone” pattern. There is no evidence of mucin production or glandular differentiation (111). Variable numbers of lymphocytes, eosinophils (129), and plasma cells may be present in the stroma.
Undifferentiated nasopharyngeal carcinoma (lymphoepithelioma) consists of cytologically uniform cells with ovoid vesicular nuclei, prominent nucleoli, and indistinct cell borders resulting in a syncytial pattern. Spindle cells may sometimes be present, and there may be scattered effete cells with shrunken, hyperchromatic nuclei. An inflammatory infiltrate rich in lymphocytes and occasionally containing prominent eosinophils (129) is usually a component of lymphoepithelioma, but the diagnosis is based solely on the nature of the neoplastic epithelial component. The inflammatory and neoplastic elements in lymphoepithelioma may interact to produce two microscopic patterns named for their initial describers (130,131).
In the Regaud pattern, the neoplastic cells form welldefined, cohesive cell nests and cords separated by inflammation (Fig. 21.31). The cohesive nature of the cell nests allows for its ready recognition as a carcinoma, and diagnosis is straightforward (116). In the Schmincke pattern, however, the inflammatory component permeates the cell nests to a much greater degree, separating and isolating the carcinoma cells in a “sea” of polymorphic inflammation that is predominantly lymphoid (Fig. 21.32). Such lesions may be difficult or impossible to distinguish from lymphoma on H&E-stained sections (116,132), particularly when the lesion appears as a lymph node metastasis from an occult primary. The two patterns have no prognostic differences, and mixtures of both patterns are common. It should be noted that the histologic features of lymphoepithelioma-like carcinoma are not specific for EBV association. Histologically identical tumors can be seen throughout the body unassociated with viral agents, and this histologic pattern, in some anatomic sites, such as oropharynx and uterine cervix, may be associated with HPV (133).
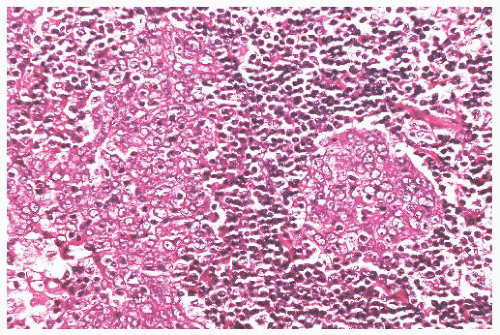 FIGURE 21.31 In the Regaud pattern of undifferentiated carcinoma (lymphoepithelioma), the neoplastic cells form cohesive nests. Confusion with lymphoma is unlikely. |
The use of immunohistochemical markers to distinguish undifferentiated carcinoma from lymphoma is discussed in
the section on lymphoplasmacytic proliferations (Figs. 21.33 and 21.34). Standard light microscopic features are also useful in this regard. Even in Schmincke pattern tumors, isolated, delicate cytoplasmic strands can be seen connecting separated cells, and a careful search should be made for more cohesive cell nests. Lymph node metastases with the Schmincke pattern can mimic Hodgkin disease in terms of both clinical characteristics (because of their typical bilaterality and presence in young patients) and microscopic features (because the neoplastic cells somewhat resemble mononuclear Reed-Sternberg cells in a polymorphic inflammatory background) (116,132). In contrast to Reed-Sternberg cells, undifferentiated carcinoma cells (a) are always mononuclear, (b) have thin chromatin rims and smaller nucleoli, and (c) lack pericellular clearing (lacunae) (116). Verrucous carcinoma may rarely arise in the nasopharynx (134,135). Its clinicopathologic features are identical to those of the more common verrucous carcinomas of the larynx and oral cavity.
the section on lymphoplasmacytic proliferations (Figs. 21.33 and 21.34). Standard light microscopic features are also useful in this regard. Even in Schmincke pattern tumors, isolated, delicate cytoplasmic strands can be seen connecting separated cells, and a careful search should be made for more cohesive cell nests. Lymph node metastases with the Schmincke pattern can mimic Hodgkin disease in terms of both clinical characteristics (because of their typical bilaterality and presence in young patients) and microscopic features (because the neoplastic cells somewhat resemble mononuclear Reed-Sternberg cells in a polymorphic inflammatory background) (116,132). In contrast to Reed-Sternberg cells, undifferentiated carcinoma cells (a) are always mononuclear, (b) have thin chromatin rims and smaller nucleoli, and (c) lack pericellular clearing (lacunae) (116). Verrucous carcinoma may rarely arise in the nasopharynx (134,135). Its clinicopathologic features are identical to those of the more common verrucous carcinomas of the larynx and oral cavity.
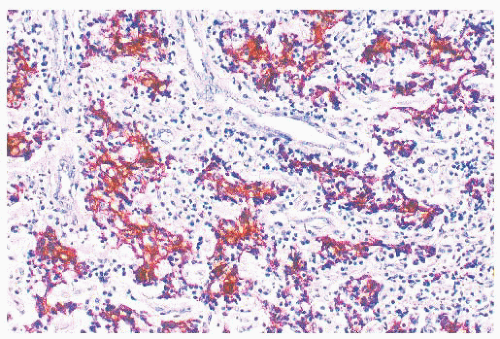 FIGURE 21.33 Undifferentiated carcinoma. In contrast to Figure 21.34, the larger neoplastic cells stain for cytokeratin, and the inflammatory infiltrate is nonreactive (anticytokeratin immunoperoxidase). |
NUT Midline Carcinoma
A distinctive and highly aggressive variant of squamous cell carcinoma has been relatively recently described that is associated with a specific genetic translocation involving the NUT gene on chromosome 15 fusing with BRD4 or BRD3 genes on chromosomes 19 or 9, respectively (136,137). These tumors, as their name implies, tend to involve midline structures, and the sinonasal region is a relatively common site. Although first described in children, they are now known to occur in patients of virtually any age.
Histologically, NUT midline carcinomas are usually composed of sheets of undifferentiated cells. Large areas of coagulative necrosis may be present. The cells have little amphiphilic or eosinophilic cytoplasm. Nuclei have irregular contours, although they tend to be somewhat uniform in size, with fine to vesicular chromatin and prominent nucleoli (Fig. 21.35). Mitotic figures and apoptotic bodies are numerous. Squamous differentiation may be seen and does not necessarily predict the partner gene (see the following discussion). The differentiation may be subtle with slight streaming of the malignant cells or it can be very distinct, with nests of maturing squamous cells and extracellular keratin formation. Some authors, including ourselves, have noted that the keratinization may appear abrupt, with sheets of immature cells juxtaposed to well-differentiated, mature squamous nests (Fig. 21.36). The well-differentiated areas may even become cystic.
NUT midline carcinomas react with antibodies to keratins and epithelial membrane antigen (EMA). CD34 immunoreactivity was seen in a little more than half of the cases studied in one series, a unique finding for an epithelial malignancy. Immunoreactivity with other antigens expressed in small blue cell tumors of childhood has not been noted, and tumors have been reported to be nonreactive with antibodies to desmin, myoglobin, smooth muscle actin, muscle actin, chromogranin, synaptophysin, leukocyte common antigen, placental alkaline phosphatase, S-100 protein, α-fetoprotein, neuron-specific enolase, CD57, CD99, and HMB-45. Evidence of Epstein-Barr virus RNA (EBER) or HPV DNA has not been identified by
fluorescence in situ hybridization (FISH). A monoclonal antibody to the NUT protein has been developed. Using 50% nuclear staining as a cutoff, the antibody showed a sensitivity of 87% and specificity of 100% (138).
fluorescence in situ hybridization (FISH). A monoclonal antibody to the NUT protein has been developed. Using 50% nuclear staining as a cutoff, the antibody showed a sensitivity of 87% and specificity of 100% (138).
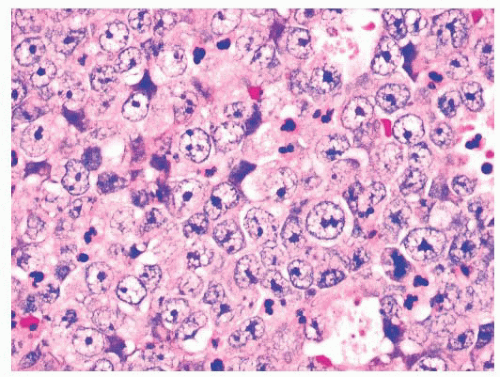 FIGURE 21.35 NUT midline carcinoma. The neoplastic cells of NUT midline carcinoma often exhibit relatively uniform nuclear features, with large numbers of scattered apoptotic cells. |
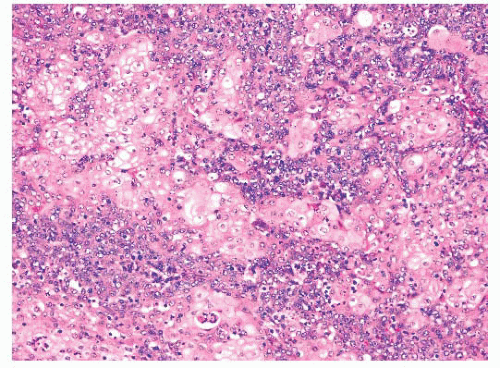 FIGURE 21.36 NUT midline carcinoma. Abrupt differentiation to rather bland-appearing squamous cells is a common but not universal feature of NUT midline carcinoma. |
The differential diagnosis for NUT midline carcinomas includes other undifferentiated carcinomas and poorly differentiated (basaloid) squamous cell carcinomas. Within the upper aerodigestive tract, the differential diagnosis includes pediatric small blue cell tumors, melanoma, olfactory neuroblastoma, high-grade hematologic malignancies, endocrine carcinomas, and other undifferentiated or poorly differentiated carcinomas including SNUC and EBV-driven lymphoepitheliomas that occur most frequently in the nasopharynx.
Identification of clear-cut epithelial differentiation rules out many of the tumors in the differential (although one should remember that some mesenchymal tumors can rarely show epithelial differentiation, e.g., primitive neuroectodermal tumor). For tumors without obvious differentiation seen histologically, immunohistochemistry can be used. Epithelial differentiation can almost always be demonstrated with pankeratin immunococktail. Negative results for other antigens such as S-100 protein, CD45, muscle antigens, neuroendocrine antigens, and germ cell antigens are also helpful as cytokeratin or EMA immunoreactivity can be seen, at least focally, with many of these tumors. Immunoreactivity for p63 is also helpful as it points to squamous differentiation and was seen in almost all of the tumors that we tested. Reactivity makes some diagnoses such as SNUC much less likely.
As noted earlier, NUT midline carcinomas have not been associated with carcinoma-related viruses such as EBV and HPV. Thus, positive in situ hybridization for these viruses excludes the diagnosis. Surrogate HPV markers such as p16 have not been studied.
There is no established treatment for these tumors. Most patients have received combination multidrug chemotherapy and radiation. Only occasional cases have undergone subsequent resection because of the high stage at presentation and because of the dismal results of multimodality treatment. Almost all patients with adequate follow-up have died of disease with an average survival time of 9 to 10 months.
VARIANTS OF ADENOCARCINOMA
Adenocarcinomas account for approximately 10% to 20% of all sinonasal malignancies (98,139,140). These tumors can be divided into two main groups: salivary-type neoplasms and a heterogeneous group of nonsalivary adenocarcinomas.
SALIVARY-TYPE ADENOCARCINOMAS
Of the salivary-type tumors, adenoid cystic carcinoma is most common in the sinonasal and nasopharyngeal region (56). The maxillary antrum is the most typical site (141), but involvement of the nasal cavity, nasopharynx, and ethmoid and sphenoid sinuses is also typical (141,142). Frontal sinus tumors are unusual. Mucoepidermoid carcinoma is the second most common salivary-type adenocarcinoma of the upper aerodigestive tract (56). The morphologic appearance and clinical behavior of these tumors are discussed in more detail in the chapter on salivary gland neoplasms (Chapter 20).
NONSALIVARY ADENOCARCINOMAS
Adenocarcinomas of the sinonasal region that do not resemble salivary-type neoplasms may be subdivided into intestinal-type and nonintestinal-type tumors. The nonintestinal tumors can be further subdivided into low-grade (56,96,143) and highgrade variants (143,144). Attempts have been made to subdivide the intestinal-type tumors as well. The clinicopathologic features of each group differ markedly, warranting careful distinction. Additionally, low-grade papillary adenocarcinomas have been described that arose from the surface epithelium of the nasopharynx (145). These subtypes are described in the following sections.
NONINTESTINAL ADENOCARCINOMAS
Low-Grade Adenocarcinoma
Clinical Features.
Low-grade sinonasal adenocarcinomas affect males and females with equal frequency and occur in a broad age range (age range, 9 to 75 years; median age, 54 years) (56,96). The distribution of lesions in one study was as follows: nasal cavity, 22%; nasal septum, 18%; ethmoid or nasoethmoid, 30%; maxillary or nasomaxillary, 13%; and multiple locations, 18% (56). There is no known association with carcinogens. Patients with low-grade carcinoma have a good prognosis. Recurrences develop in 30% of cases but do not indicate intractable disease (56). In one study, after a median follow-up interval of more than 6 years, 78% of patients were disease free (56). Death from disease is a result of uncontrollable local invasion rather than metastases. None of the nine patients with lowgrade nasopharyngeal adenocarcinoma reported by Wenig et al. (145) experienced metastases, although one tumor recurred locally after radiation therapy.
Morphologic Characteristics.
Low-grade sinonasal adenocarcinomas are a morphologically heterogeneous group. In some, the architectural and cytologic uniformity frequently leads to misdiagnosis as an adenoma or a papilloma (145). In the majority of cases, small glands are lined by a single layer of cuboidal or columnar cells, often in a back-to-back arrangement without intervening stroma (Figs. 21.37 and 21.38) (56).
Some glands are cystically dilated, and others contain papillary infoldings. Nuclei vary in size from case to case but tend to be uniform within a given lesion. Mitotic figures are generally rare but may occasionally be abundant (56). Most tumors contain both intracellular and extracellular mucin, and they can be seen to originate from surface mucosa. Some neoplasms included under the rubric of low-grade adenocarcinoma consist of cells with basophilic cytoplasm in small, acinar-like nests. Such lesions are indistinguishable from acinic cell carcinoma of the salivary gland type (56) and should probably be segregated from the other, nonsalivary tumors in this group.
Some glands are cystically dilated, and others contain papillary infoldings. Nuclei vary in size from case to case but tend to be uniform within a given lesion. Mitotic figures are generally rare but may occasionally be abundant (56). Most tumors contain both intracellular and extracellular mucin, and they can be seen to originate from surface mucosa. Some neoplasms included under the rubric of low-grade adenocarcinoma consist of cells with basophilic cytoplasm in small, acinar-like nests. Such lesions are indistinguishable from acinic cell carcinoma of the salivary gland type (56) and should probably be segregated from the other, nonsalivary tumors in this group.
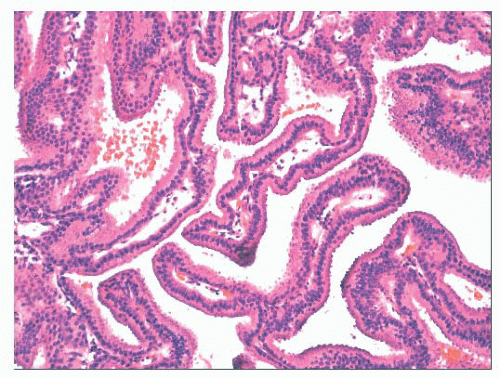 FIGURE 21.37 Low-grade sinonasal adenocarcinoma forms papillary structures lined by uniform, bland cells. |
Low-grade sinonasal adenocarcinoma must be distinguished from intestinal-type adenocarcinomas, which are described in the next section, because of the more aggressive clinical course of the latter lesion. Making the distinction is usually straightforward, given the nuclear stratification and colonic appearance of the intestinal neoplasms. In addition, intestinal-type tumors are more pleomorphic than low-grade adenocarcinomas on cytologic examination—with the exception of rare nasal neoplasms resembling normal intestinal mucosa. Staining for CDX-2 and other intestinal markers in the intestinal-type tumors may aid in this distinction. Oncocytic schneiderian papillomas may also be confused with low-grade adenocarcinoma. Heffner et al. (56) list the following differentiating features: (a) stratified epithelium in papillomas as opposed to singlelayered cells in adenocarcinoma; (b) true glandular lumina in adenocarcinoma; and (c) more abundant, myxomatous stroma in papillomas. The complex papillary pattern, vesicular nuclei, and focal psammoma bodies seen in some low-grade nasopharyngeal adenocarcinomas can mimic a metastatic papillary carcinoma of the thyroid gland (145). However, the primary nasopharyngeal neoplasms lack positivity for thyroglobulin (145). Low-grade adenocarcinomas have been noted to arise in and potentially be confused with REAHs (96).
High-Grade Adenocarcinoma
Clinical Features.
Nonintestinal-type high-grade adenocarcinomas of the sinonasal region are a heterogeneous group of tumors that cannot be classified as salivary gland-type adenocarcinomas; do not have an intestinal phenotype; and have prominent cellular pleomorphism, a high mitotic rate, and often prominent necrosis (143,144). The majority has developed in men, and patients have ranged in age from adolescence to the elderly. Bulky disease with involvement of both the nasal cavities and paranasal sinuses at presentation is typical. In spite of aggressive therapy, more than half of the people with these tumors have died of disease (146).
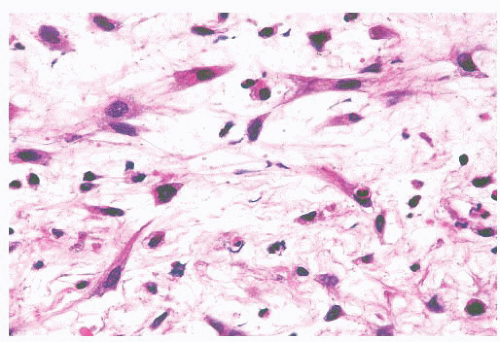
Morphologic Characteristics.
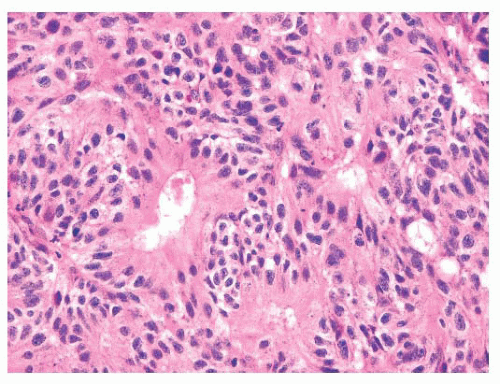
These tumors are histologically heterogeneous (143,144) (Figs. 21.39 and 21.40). Some resemble the blastomatous component of teratocarcinosarcoma, have solid and trabecular growth with occasional small cystic spaces, and are composed of neoplastic cells that have a small amount of amphiphilic cytoplasm. Occasional cases are composed of cells with more abundant eosinophilic cytoplasm somewhat resembling salivary duct carcinoma. Many examples
will have focally solid growth resembling SNUC. Rare cases have been composed of clear cells and resembled renal cell carcinoma (144). Some high-grade adenocarcinomas somewhat resemble high-grade mucoepidermoid carcinomas and have been noted to arise within oncocytic schneiderian papillomas (144).
will have focally solid growth resembling SNUC. Rare cases have been composed of clear cells and resembled renal cell carcinoma (144). Some high-grade adenocarcinomas somewhat resemble high-grade mucoepidermoid carcinomas and have been noted to arise within oncocytic schneiderian papillomas (144).
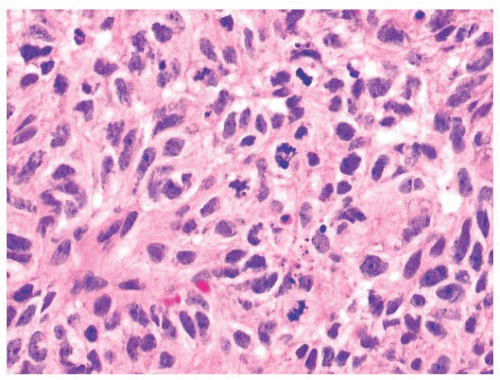 FIGURE 21.40 High-grade nonintestinal adenocarcinoma. In other areas, the tumor shown in Figure 21.35 grows as more solid sheets of epithelioid to spindle-shaped cells with a very high mitotic rate. |
In keeping with their nonintestinal phenotype, these tumors lack CDX-2 and generally lack CK20 immunoreactivity (144). Diffuse, strong CK7 immunoreactivity has been seen in nearly half of cases. S-100, p63, and neuroendocrine antigen immunoreactivity are only rarely seen.
High-grade nonintestinal sinonasal adenocarcinomas must be distinguished from their low-grade counterparts as they have a markedly different prognosis. Low-grade lesions should be predominately papillary or tubular unlike high-grade lesions which are frequently solid or trabecular. Although cytologic atypia may be focally present in low-grade lesions, abundant mitotic figures or tumor necrosis is diagnostic of a high-grade lesion.
High-grade nonintestinal adenocarcinomas should also be distinguished from intestinal-type adenocarcinomas. Most sinonasal intestinal-type adenocarcinomas are obviously gland forming and frequently produce abundant extracellular mucus. Immunohistochemistry may be helpful, especially with small biopsies. Intestinal-type sinonasal adenocarcinomas are immunoreactive with antibodies to CK20, CDX-2, and MUC-2, whereas high-grade, nonintestinal adenocarcinomas rarely, if ever, express these markers.
As noted earlier, some high-grade adenocarcinomas may partially resemble SNUC. We define SNUC as a tumor which, as the name indicates, shows no evidence of glandular (or squamous) differentiation, although others have allowed for limited differentiation in these tumors. The distinction of these high-grade adenocarcinomas from salivary duct carcinoma also can be very difficult and, indeed, may be semantic.
We have seen occasional high-grade adenocarcinomas that expressed p16 and contained high-risk types of HPV by in situ hybridization. Some of these have had focal p63 immunoreactivity and could be considered as adenosquamous carcinomas, although they differ from the typical adenosquamous carcinomas that have obvious intracellular mucin accumulation. The tumors typically have had a trabecular and cystic growth, lined focally with tall columnar cells.
INTESTINAL-TYPE ADENOCARCINOMA
Clinical Features
After adenoid cystic carcinoma, the second most common glandular neoplasm of the sinonasal region is composed of cells mimicking normal, adenomatous, or carcinomatous intestinal mucosa (56,147,148,149,150,151,152 and 153). Although the histologic spectrum is broad, as described later, the clinical features are stereotypical and are only slightly affected by the microscopic form of intestinal differentiation. Nasal intestinal-type adenocarcinoma has a strong association with long-term exposure to fine hardwood dusts in the woodworking industry (152,154,155,156 and 157); in such populations, prior to the advent of appropriate dust control measures, the incidence approaches 1000 times that of the general public (154). Approximately 20% of cases occur in patients with industrial wood-dust exposure. Exposure to leather dust has also been incriminated (158). With the advent of widespread dust control measures, the incidence of occupationally related tumors has declined dramatically.
There are minor clinical differences between intestinaltype adenocarcinomas occurring sporadically and those arising in woodworkers (147). Tumors related to industrial dust exposure are not surprisingly found predominantly in men (85% to 95%), show a striking predilection for the ethmoid sinus (156,157), and have a slightly better prognosis (50% at 5 years) (157). Tumors arising sporadically frequently develop in women, often arise in the maxillary antrum (20% to 50%), and have a poorer prognosis (20% to 40% at 5 years) (147). In both groups, the clinical course may be quite protracted; 5-year survival does not indicate cure. Local recurrences are common (53%) (147), and death usually results from uncontrollable local disease with intracranial extension or exsanguination. Metastasis to regional lymph nodes takes place in approximately 8% of cases, and distant metastases are seen in approximately 13% of cases (147).
Although all forms of intestinal-type neoplasia in the sinonasal region are at least locally aggressive, studies have suggested that grading these lesions provides additional prognostic information (159,160 and 161). Kleinsasser and Schroeder (159) divided intestinal-type sinonasal adenocarcinomas into the papillarytubular type, grades I to III; the goblet cell type; the signet ring cell type; and the mixed or transitional type. Their study, and the subsequent studies by Franquemont et al. (160) and Franchi et al. (161), showed a somewhat better prognosis for the well-differentiated papillary-tubular neoplasms.
Morphologic Characteristics
These neoplasms recapitulate the entire range of appearances assumed by normal and neoplastic large and small intestinal mucosa. At the exquisitely well-differentiated end of the spectrum are tumors that resemble normal intestinal mucosa replete with goblet, resorptive, Paneth, and argentaffin cells, along with well-formed villi and muscularis mucosae (148,153). Although it is tempting to label such proliferations as benign heterotopias, they are locally aggressive, invasive lesions (153).
The papillary tumors consist of elongated fronds lined by stratified columnar and goblet cells reminiscent of intestinal villous or tubular adenoma (Fig. 21.41). Papillary tumors may be invasive or intramucosal (147,150).
The papillary tumors consist of elongated fronds lined by stratified columnar and goblet cells reminiscent of intestinal villous or tubular adenoma (Fig. 21.41). Papillary tumors may be invasive or intramucosal (147,150).
 FIGURE 21.41 Intestinal-type sinonasal adenocarcinoma has a papillary configuration resembling adenomatous polyp of the colon. |
The most common form of sinonasal intestinal-type adenocarcinoma resembles conventional colonic adenocarcinoma (Fig. 21.42). In this variant, glands lined by more pleomorphic columnar cells are often back to back, vary in size, and invade the underlying stroma. Intracellular mucin is present focally, but goblet cells are not prominent (147,150). In less differentiated tumors, solid sheets of tumor cells may be present, with only scattered glandular lumina (147). Completing the analogy to intestinal neoplasms are the less typical mucinous tumors (147,150). The predominant pattern in this variant consists of large glands distended with mucin or pools of extracellular mucin containing small clusters of neoplastic cells (Fig. 21.43). Signet ring cells form a minor component or, rarely, predominate (150). The resemblance of intestinal-type adenocarcinoma to normal and neoplastic intestinal epithelium is not limited to the light microscopic manifestations. Ultrastructural studies have confirmed the presence of resorptive, goblet, Paneth, and argentaffin cells identical to their intestinal counterparts (153,162,163), and intestinal-type hormones have been documented on immunocytochemical evidence (162). Markers of intestinal differentiation, CDX-2 and MUC-2 (Fig. 21.44), are positive in these tumors (164). Although these markers are of value in the distinction from low-grade adenocarcinoma, they do not establish primary sinonasal origin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree