CHAPTER 3 Normal bone marrow histology
Normal bone marrow – generalities and function
The term ‘bone marrow’ (BM) refers to the tissue occupying the cavities under the cortex within the honeycomb of trabecular bone. Normal marrow is either red, consisting of the hematopoietic tissue, or yellow, composed mainly of fat cells (adipose tissue). In children most bones contain hematopoietic marrow, almost to the exclusion of fat cells. In the adult, red marrow is found in the skull, sternum, scapulae, vertebrae, ribs, pelvic bones and the proximal ends of the long bones (e.g. femora and humeri). The hematopoietic marrow produces the mature blood cells, which have a finite life span and must be constantly replaced (see Chapter 2). The weight of the total BM is 1600–3700 g. The BM is composed of:
Bone marrow trephine biopsy
The process of obtaining a bone marrow trephine biopsy (BMTB) originates in the ancient procedure of trepanning.1 Prior to the advent of BMTB needles, clot preparations of aspirated marrow were prepared for diagnostic purposes. BM biopsies were obtained only as a means of diagnosis if marrow was inaspirable, a ‘dry tap’. The modification of needles by Jamshidi in the 1970s revolutionized the process of obtaining intact cores of bone and bone marrow for examination, primarily from the pelvis. The most common site biopsed is the posterior superior iliac crest. Other sites which can be biopsied include the anterior superior iliac crest, tibia, and vertebrae. Biopsy of the sternum is contraindicated due to the significant morbidity and mortality associated with this practice. The optimal length of a BMTB is 2–3 cm; shorter biopsies may not be representative and may not detect diseases that have a focal or patchy pattern of BM involvement.2
Processing and stains
A number of methods are available for fixation, decalcification and embedding of the BMTB2 (Table 3.1). For optimal evaluation, sections are cut at a thickness of 1–3 µm. Conventional stains are hematoxylin and eosin (H&E) (Fig. 3.1A) and Giemsa (Fig. 3.1B) stains. Giemsa staining generally gives better discrimination of cell types based on cytoplasmic staining characteristics, that is:
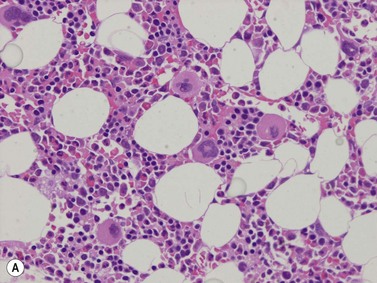
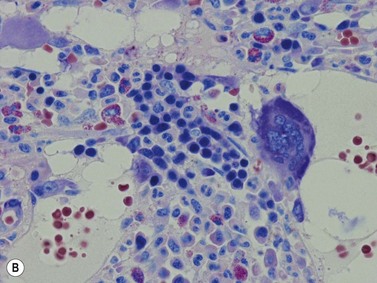
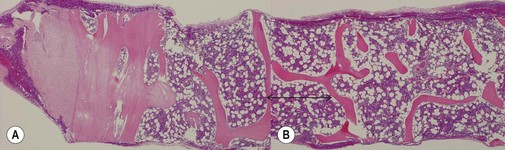
Fig. 3.1 (A, B) Normal bone marrow biopsy showing normal marrow cellularity and architecture. (A) Hematoxylin & Eosin stain. × 200. (B) Giemsa stain. × 400.
Other stains commonly used include the Perls stain for iron, silver stains for reticulin and Masson’s trichrome stain or Martius scarlet blue stain for collagen. Immunohistochemistry (IHC) using monoclonal or polyclonal antibodies, and in situ hybridization using nucleic acid probes can be used to identify specific cell types and genetic aberrations; these are discussed in sections below.3
Marrow cellularity
The BMTB is particularly useful for the assessment of marrow cellularity. This is the relative amount of BM cells to adipocytes, which is assessed subjectively and should be interpreted in the context of the age of the patient. The terms normocellular (normal for age), hypercellular (increased cellularity for age) and hypocellular (reduced cellularity for age) are used. Cellularity reduces with increasing age (Table 3.2).4,5 In practice, the formula (cellularity = 100 − patient age) can be applied for adults; however, it does not correlate with cellularity at the extremes of the age range. The intertrabecular spaces adjacent to the marrow cortex tend to be hypocellular and should not be assessed when determining overall BM cellularity.
Table 3.2 Cellularity ranges for various age groups
| Age | Cellularity |
|---|---|
| Newborn to 3 months | 80–100% |
| Childhood | 60–80% |
| 20–40 years | 60–70% |
| 40–70 years | 40–50% |
| >70 years | 30–40% |
Marrow architecture
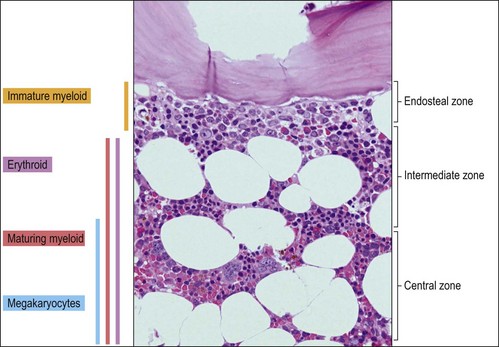
The BMTB enables the assessment of bone marrow architecture, the distribution of cellular elements and the bone and stromal cells. The outermost elements of the biopsy are composed of collagenous periosteal connective tissue, followed by a zone of cartilage or cortical bone (depending on the age of the patient). After this the bone breaks up into a meshwork of trabeculae, between which are the intertrabecular spaces. Hemopoietic cells are present within these intertrabecular spaces and are supported by fat cells, stromal cells, histiocytes extracellular matrix and blood vessels (Fig. 3.2). The hemopoietic cells are located within the intertrabecular spaces. The intertrabecular areas can be divided into three zones which contain different hemopoietic cell types (Fig. 3.3):
Hemopoiesis
The process of formation of blood elements from hemopoietic stem cells has been described in detail in Chapter 2. The BMTB enables the visualization of the spatial localization of the individual cell lineages during their development. Hemopoietic progenitors are present in cords, islands or clusters. Fully mature erythroid and granulocytic cells and platelets migrate through the sinusoidal endothelial cells to enter the bloodstream.
Erythropoiesis
Erythroid progenitors are found in small and large ‘islands’ called erythroid colonies within the intermediate and central zones of the marrow cavity. Erythroid islands are made up of concentric circles of immature erythroblasts (proerythroblasts) and a spectrum of maturing erythroid precursors leading to the late erythroblasts. Each erythroid island has a central iron-containing macrophage. The most primitive erythroid progenitor cells are present centrally around the macrophage and the maturing forms towards the periphery6 (Fig. 3.4). The central macrophage possesses dendritic processes, which extend between the maturing erythroid precursors. Its function is to support and nurture the erythroblasts, act as a source of iron and remove debris from dying cells and extruded nuclei. The central macrophage is often difficult to identify in histologic sections. Erythroid precursors are easily identified by being in distinct islands with cells of varying maturity, their almost perfectly round nuclei and by a perinuclear halo, an artifact of fixation and processing.
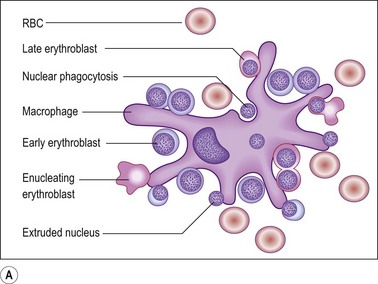
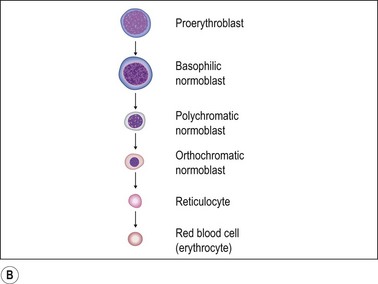
Fig. 3.4 (A, B) Erythropoiesis: organization of the erythroid colony and stages of erythroid maturation.
((A) Modified from Erythroblastic islands: niches for erythropoiesis. Blood 2008; 112: 470; (B) http://www.healthsystem.virginia.edu/internet/haematology/hessimages/erythropoiesis.jpg.)
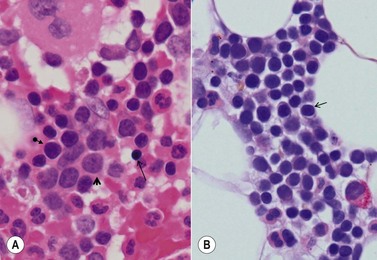
Proerythroblast. The earliest recognizable erythroid precursors (proerythroblasts) are medium to large round cells with minimal cytoplasm, large round nuclei with dispersed or open chromatin, many small nucleoli and a crisp nuclear membrane. A rim of weakly basophilic cytoplasm with a halo is also present (Fig. 3.5A).
Maturing erythroblast (also called normoblast). These are smaller than proerythroblasts, and differ in their nuclear and cytoplasmic characteristics. As a rule, with maturation, nuclear size reduces and the amount of cytoplasm increases. The nuclear chromatin becomes more condensed and acquires a uniform, condensed, hyperchromatic ‘ink dot’ appearance. It is this nuclear characteristic that enables late normoblasts to be distinguished from lymphocytes. As hemoglobin forms, the cells acquire rims of pale pink cytoplasm (orthochromatic erythroblast) which with further maturation acquires the crisp orangiophilia of mature RBCs (Fig. 3.5B).
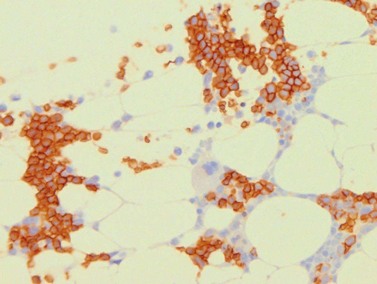
Erythroid cells can be identified by IHC using antibodies to glycophorin A (CD235) or C and intracellular hemoglobin. Glycophorin A highlights both nucleated erythroid precursors and RBCs (Fig. 3.6) while hemoglobin A tends to be restricted to hemoglobinized nucleated erythroid precursors. In situ hybridization can also be performed using probes for hemoglobin A.
Granulopoiesis
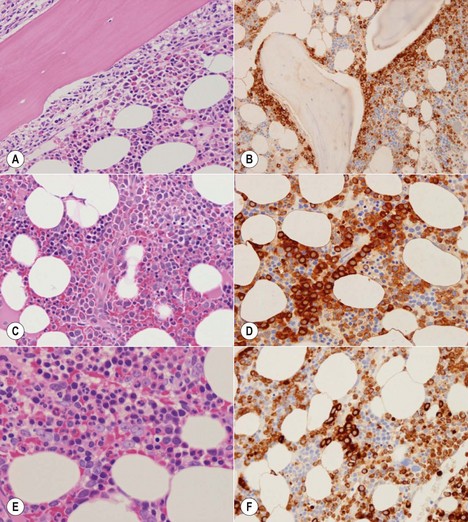
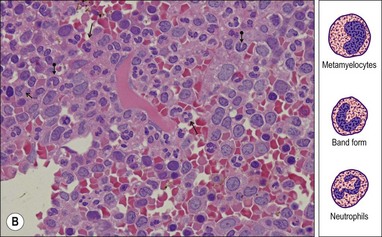
The granulocytic series consists of neutrophils, eosinophils, basophils and mast cells. All the morphologic stages of myeloid maturation as seen in the BM aspirate can be identified on trephine sections. Most immature granulocytic cells (myeloblasts and promyelocytes) are arranged along the endosteal surface (paratrabecular zone) (Fig. 3.7A, B) or as periarteriolar cuffs (Fig. 3.7C, D); these constitute the granulocytic ‘generation zones’; however, precursors are also scattered throughout the rest of the marrow as is often seen on myeloperoxidase (MPO) staining (Fig. 3.7E, F). Maturing granulocytic cells occur in the intermediate and central intertrabecular zones.
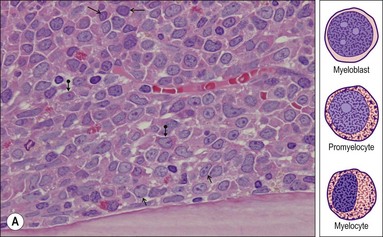
Myeloblast. This is the earliest recognizable granulocytic cell in the BMTB. It is a medium sized cell with a centrally placed round-ovoid nucleus, with very open, pale-staining chromatin which contains one or more fine eosinophilic nucleoli. A small amount of cytoplasm is often present; granules are difficult to identify (Fig. 3.8A).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree