and Natalia Buza1
(1)
Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
Keywords
Nonneoplastic lesions of the ovaryCystic follicle/follicle cystCystic corpus luteum/corpus luteum cystEndometriosis/endometriotic cystAtypical endometriosisStromal hyperthecosisTubo-ovarian abscessMiscellaneous primary ovarian tumorsIntroduction
Nonneoplastic—physiologic or reactive—conditions of the ovary may present as a mass lesion and mimic a malignant process clinically or radiologically and may result in a diagnostic dilemma on frozen section examination. Familiarity with the clinical context, the spectrum of frozen section histomorphology, and the potential technical artifacts can help avoiding overinterpretation of these benign lesions. Precise classification of benign findings, however, is rarely required for intraoperative management.
This chapter, in addition to presenting a brief overview of the most common nonneoplastic ovarian entities, also includes a few rare primary ovarian tumors, i.e., lymphomas and small cell carcinomas.
Nonneoplastic Lesions of the Ovary
Cortical Inclusion Cysts
Very common, typically incidental findings beneath the ovarian surface.
Measure <1 cm in size.
Single layer of epithelial cell lining, may be tubal type/ciliated or flattened (Fig. 11.1).
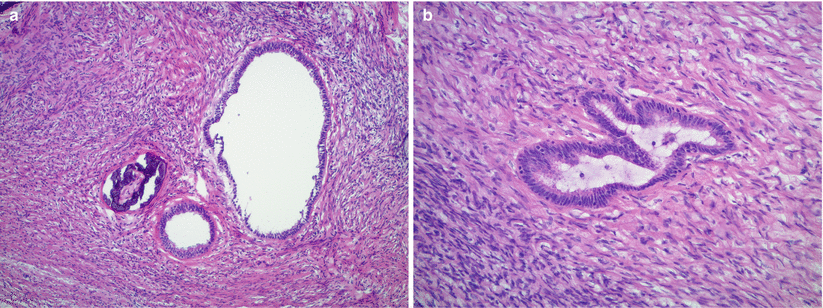
Fig. 11.1
Cortical inclusion cysts in the ovarian stroma with focal calcification (a). The lining epithelium is composed of a single layer of bland, flattened, or tubal-type cells (b)
Mild cytological atypia may be seen.
May be associated with calcifications.
No papillary epithelial proliferation or tufting.
Diagnostic pitfalls/key intraoperative consultation issues
Cortical inclusion cysts with prominent calcification and mild epithelial atypia may raise suspicion for borderline/atypical proliferative serous tumor; however, they lack epithelial proliferation, or tufting.
Cystic Follicle/Follicle Cyst
May occur at any age, although most common during the reproductive years.
Lesions measuring less than 3 cm are typically designated as cystic follicle, while follicle cysts usually range between 3 and 8 cm in size.
Cut surface shows a thin cyst wall, smooth inner surface, and clear or hemorrhagic fluid contents.
Microscopically the cysts are lined by variable number of granulosa cell layers (Fig. 11.2).
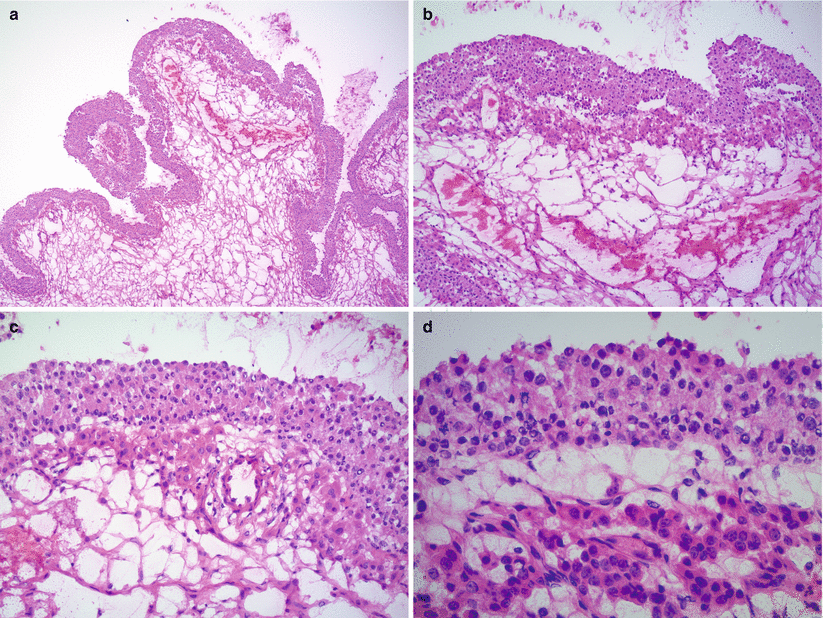
Fig. 11.2
Cystic follicle/follicle cyst. The cyst is lined by multiple layers of granulosa cells (a, b), characterized by uniform, round nuclei and often luteinized, eosinophilic cytoplasm (b, c). Luteinized theca cells are also present under the granulosa cell layer (b–d). Occasional mitotic figures may be seen among the granulosa cells (d)
Uniform, round to oval nuclei and small nucleoli.
Mitotic figures may be present.
Often luteinized with abundant eosinophilic cytoplasm.
Outer layer of luteinized theca cells is often seen.
Differential diagnosis
Adult granulosa cell tumor (AGCT)
Juvenile granulosa cell tumor (JGCT)
Diagnostic pitfalls/key intraoperative consultation issues
AGCT may be unilocular, cystic, mimicking a follicle cyst.
Luteinization is uncommon in cystic AGCT, while it is often seen in follicle cysts.
Cystic growth pattern and cytological features of JGCT show similarity to those of follicle cysts.
Follicle formation within the cystic granulosa cell lining is characteristic of JGCT [see Chap. 10] and not a feature of follicle cyst.
Cystic Corpus Luteum/Corpus Luteum Cyst
Enlarged, cystic corpus luteum is common during the reproductive years.
Patients may present with abdominal pain, menstrual abnormalities, and rarely hemoperitoneum due to rupture [1].
Yellow, cystic cut surface often with central hemorrhage.
Thick, undulating lining composed of large luteinized granulosa cells (Fig. 11.3).
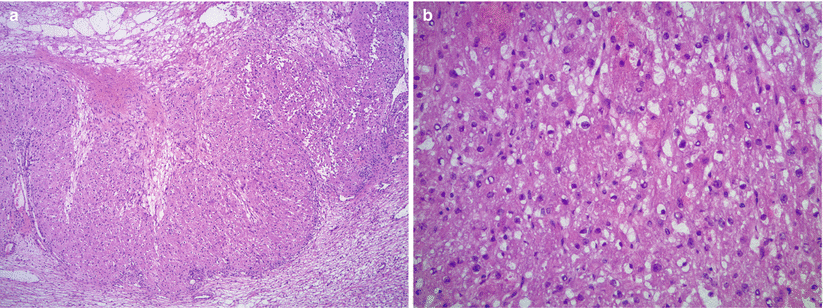
Fig. 11.3
Cystic corpus luteum/corpus luteum cyst. The cyst has thick, undulating layers of granulosa cells with relatively uniform, round nuclei and abundant eosinophilic cytoplasm (a). Cytoplasmic vacuolization may be seen on frozen sections (b)
Uniform, round nuclei with small nucleoli
Abundant eosinophilic cytoplasm—may appear vacuolated on frozen sections
Differential diagnosis
Luteinized follicle cyst
Steroid cell tumor
Leydig cell tumor
Diagnostic pitfalls/key intraoperative consultation issues
Corpus luteum cyst is usually easily recognized on frozen sections.
Distinction between corpus luteum cyst and other benign entities—e.g., luteinized follicle cyst—is not critical for intraoperative management.
Steroid and Leydig cell tumors lack the characteristic undulating arrangement of cells.
Cystic change is not a feature of steroid or Leydig cell tumors.
Reinke crystals are absent in corpus luteum cyst.
Endometriosis/Endometriotic Cyst
Endometriosis often involves the ovaries and may present with pelvic/abdominal mass and/or pain, irregular menstruation, dysmenorrhea, and infertility.
Ovary may be significantly enlarged, often >10 cm in size.
Ovarian surface adhesions and tubo-ovarian adhesions are often present.
Cut surface may be solid, hemorrhagic, or cystic, filled with red-brown fluid (“chocolate cyst”) (Fig. 11.4).
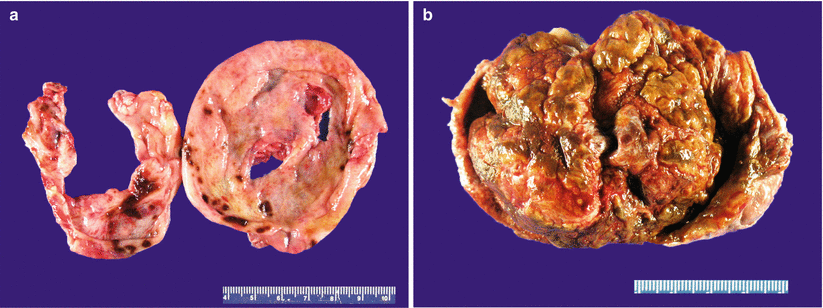
Fig. 11.4
Ovarian endometriosis. The lesion may appear grossly as a hemorrhagic cyst with a smooth, focally red-brown lining (a) or as a predominantly solid, hemorrhagic, tan-brown mass (b)
Cystic lesions may have intraluminal solid/polypoid protrusions—intraoperative frozen section sampling from these areas is important.
Microscopic examination shows endometrial glandular epithelium and endometrial stroma often with abundant hemosiderin-laden macrophages (Fig. 11.5).
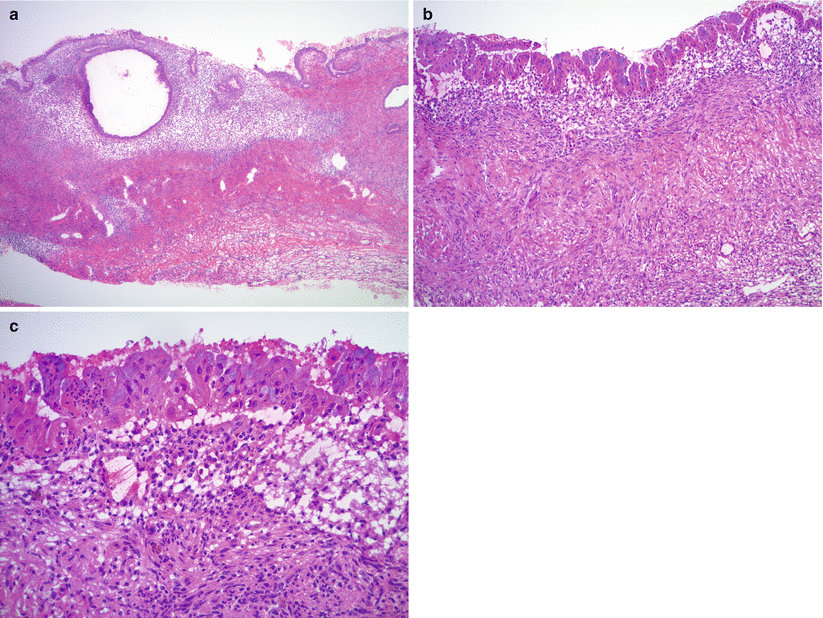
Fig. 11.5
Ovarian endometriosis. Histologically the lesion is characterized by variable proportions of endometrial stroma and endometrial glands/glandular epithelium (a). Note the distinctly different microscopic features of endometrial versus ovarian stroma (b). Mucinous metaplasia is common within the endometrial epithelium (b, c)
Characteristic vascular network of spiral arterioles in endometrial stroma helps distinguishing it from background ovarian stroma.
Epithelial lining may show metaplastic changes—tubal (ciliated), mucinous, hobnail, or eosinophilic (papillary syncytial) [2].
Cytological and/or architectural atypia may be seen—“atypical endometriosis” (Figs. 11.6 and 11.7; Fig. 11.7 clear cell carcinoma arising in atypical endometriosis).
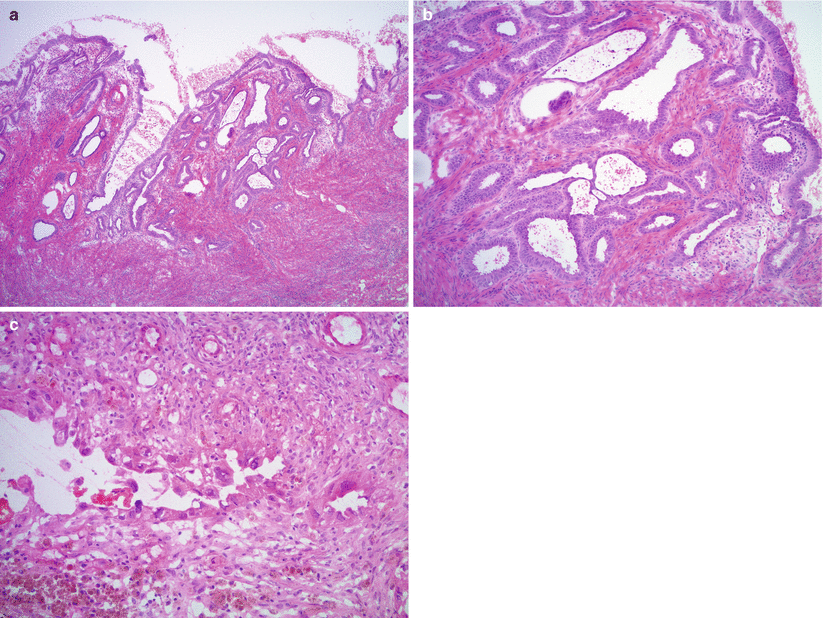
Fig. 11.6
Atypical endometriosis. Note the glandular crowding with complex architecture and atypia (a, b), similar to complex atypical hyperplasia of the eutopic endometrium, or marked cytological atypia of the endometrial glandular epithelium (c)
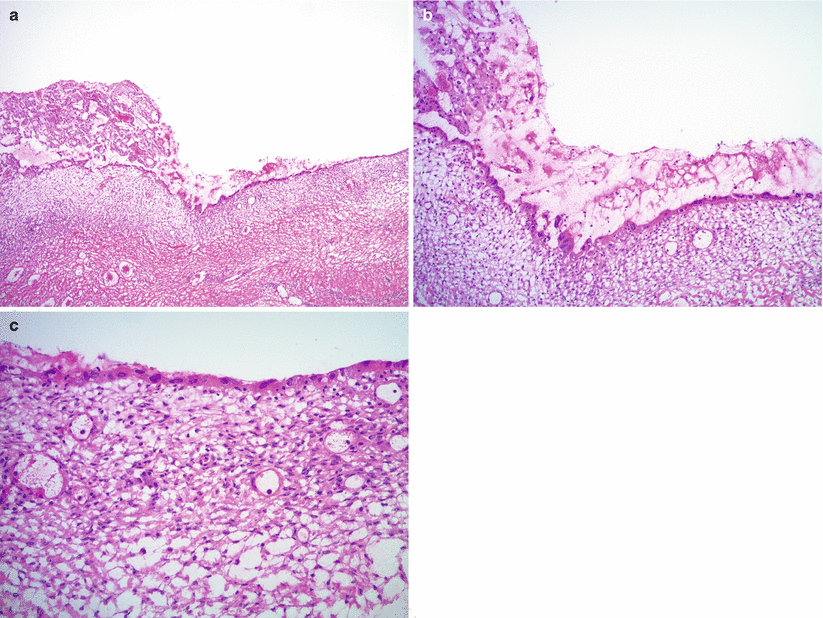
Fig. 11.7
Clear cell carcinoma of the ovary, arising in an endometriotic cyst. The endometrial epithelial lining shows marked cytological atypia (atypical endometriosis) (a, b, right side of images; c). Adjacent intraluminal tubulo-cystic proliferation represents a focus of clear cell carcinoma (a, b, left side of images)
Polypoid endometriosis resembles the growth pattern and architectural features of uterine endometrial polyps.
Differential diagnosis
Hemorrhagic follicle cyst
Clear cell carcinoma arising in endometriosis
Endometrioid adenocarcinoma arising in endometriosis
Diagnostic pitfalls/key intraoperative consultation issues
Atypical endometriosis—endometriosis with either cytological atypia or hyperplastic changes similar to eutopic (uterine endometrial) complex atypical hyperplasia—has been shown to have a benign clinical course and does not require extensive surgery [3].
Sampling for frozen section should focus on intraluminal polypoid masses and solid areas to rule out malignancy—most often clear cell and endometrioid carcinomas [see Chap. 8].
Distinction between endometriosis and other benign mimics is typically not crucial for intraoperative management.
Stromal Hyperthecosis
May occur during the reproductive years and in postmenopausal patients.
Patients may be asymptomatic or may present with endocrine manifestations (androgenic> estrogenic) [4].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


