Non-Neoplastic Maternal Gestational Diseases
Chapter 32 emphasizes the pathologic findings. This chapter focuses on the placental findings in common clinical scenarios. Points of particular relevance in the gross examination of the placenta are highlighted. While substantial overlap exists for some entities, e.g., intrauterine growth restriction (IUGR) and uteroplacental insufficiency, the scenarios are presented separately for ease of discussion.
Presentation of the findings in a fashion intelligible to clinicians is of major importance. The terminology proposed by the Society of Pediatric Pathology has been referred to.1–3 A synopsis of the findings may be given as a ‘one-liner’ in the pathology report, and may help clinicians to categorize the histologic findings (Table 33.1).
Table 33.1
Classification System for Placental Reporting
| 1 | Normal placenta |
| 2 | Placenta with chorioamnionitis |
| 3 | Placenta with villitis |
| 4 | Placenta with materno-placental circulatory disorder |
| 5 | Placenta with fetal–placental circulatory disorder |
| 6 | Placenta with maturation disturbances |
| 7 | Placenta with findings suggestive of gene aberration |
| 8 | Placenta with placentation defects |
| 9 | Placenta with other pathology |
Courtesy of Professor Borghild Roald, Oslo, Norway.4
Early Pregnancy Loss (Spontaneous Miscarriage)
Selected tissue from all miscarriages should be examined microscopically. Tissues felt to be present macroscopically are sometimes absent histologically, and the converse is also true. Fetal parts, when present, may be examined in accordance with parental wishes. Suction termination disrupts the fetus, but measurements of a hand or foot permit comparison against tables of growth rates. Whole fetuses and large embryos may be examined according to published protocols.
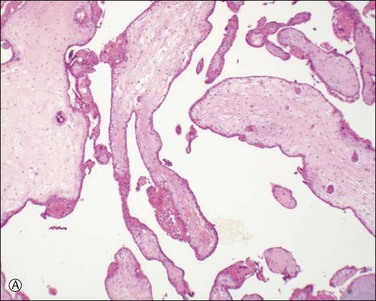
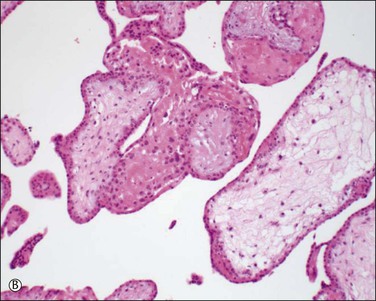
Chromosomal abnormalities in the conceptus are more frequently identified in earlier than in later fetal losses, with 65–75% of first trimester miscarriages having an abnormal karyotype.5,6 Most will be sporadic events, but a small minority result from balanced translocations in one of the parents and hence may recur. Some of the more commonly encountered karyotypic abnormalities are trisomies, especially 16 and 13, and monosomy X (45,X). Even when the karyotype is normal, there is a clear association between congenital abnormality and miscarriage. The incidence of neural tube defects and other minor abnormalities, such as cleft lip and palate or polydactyly, is higher in abortuses than in live births. Abnormalities of chromosomes 16 and 22 are prominent in early pregnancy loss (Figure 33.1A and B), and these chromosomes are preferentially involved by confined placental mosaicism.7
There have been several attempts to determine the etiology of early fetal loss by morphologic examination. Villous features thought to represent karyotypic abnormalities include vesicular change, irregular villous contour, presence of trophoblastic pseudoinclusions, trophoblastic hyperplasia, abnormal stromal cells, and, in the case of monosomy X, villous fibrosis and hypoplasia. Recognition of abnormalities at the maternal–embryonic interface (i.e., absence of interstitial trophoblast columns, absence of intravascular trophoblast plugs, and absence of physiologic changes in spiral arteries) increases the sensitivity of histology for detection of chromosomal abnormalities. Recurrent and sporadic miscarriages have similar findings, and in the majority of cases it is difficult to ascertain the cause.8 Elegant light and electron microscopic studies support the concept that many early pregnancy losses result from premature onset of the maternal–placental circulation secondary to inadequate trophoblast invasion.
Uterine abnormalities such as leiomyomas, cervical incompetence, and congenital abnormalities of müllerian duct fusion may all contribute to early pregnancy loss. Infection becomes increasingly important later in gestation as the influence of abnormal karyotype wanes. Early on, Campylobacter sp., Listeria monocytogenes, Toxoplasma gondii, rubella, cytomegalovirus (CMV), and syphilis are important. Molecular analysis suggests that coxsackie virus is one of the most common infectious agents, but placental findings are nonspecific.9 Similarly, human papillomavirus (HPV) is more commonly demonstrated in spontaneous pre-term delivery.10
The finding of a placental site trophoblastic reaction is important as it generally excludes that the pregnancy was ectopic in the fallopian tube and merely became displaced into the uterus following miscarriage. The presence of chorionic villi and decidua by themselves theoretically do not achieve this according to some reports in the literature,11 but neither of the authors has encountered an example of chorionic villi in the uterus where the origin proved to be an ectopic pregnancy. In summary, examination of the early pregnancy loss will:
Mid to Late Pregnancy Loss
If a miscarriage at 18–22 weeks is fresh, infection is a likely cause and examination should focus on the membranes. A small placenta is frequently found in trisomies and should prompt cytogenetic analysis, as karyotypic abnormalities can be expected from over 85% of placental cultures even when maceration is present.12 Second trimester miscarriages may occur following abnormal implantation, with ischemic changes up to and including infarction. Any infarction in the second trimester is likely to be clinically significant, and should be confirmed histologically. Placental infarction is associated with cerebral ischemia in the fetus, particularly in growth-restricted infants.13 The mean gestational age of pregnancy loss due to intervillositis in our practice is 25 weeks, and any increase in fibrin should prompt careful evaluation for this entity. Villitis of unknown etiology is uncommon before the third trimester.
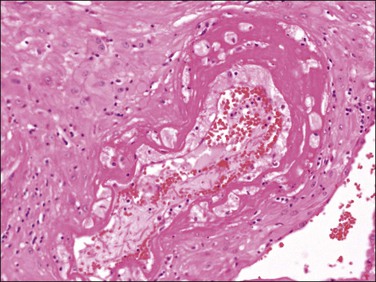
Caution must be exercised in making the diagnosis of fetal thrombotic vasculopathy in macerated fetuses, unless there is unequivocal evidence of antemortem vascular pathology, such as clearly defined avascular villi (Figure 33.2). This is usually not possible, but, where present, enables antemortem pathology to be distinguished from the secondary effects of intrauterine death.
Figure 33.2 Despite changes of intrauterine death, avascular villi can be discerned (left of picture).
Abnormal cord coiling, assessed as both under- and overcoiled cords, is associated with fetal death.14,15 Cord coiling is frequently presented as an explanation for fetal death. Features of vascular compromise such as avascular villi, vascular ectasia, and mural fibrin thrombi should be sought in this context.
The role of an individual placental finding in stillbirth may be problematic. A National Institute of Child Health and Human Development workshop in 2007 evaluated maternal, fetal, and placental conditions as a cause of stillbirth.16 The proceedings also include useful overviews of the role of infectious organisms in stillbirth.
Abruption
Abruption, a condition in which the placenta detaches if not tears away from the uterine wall, can be a dramatic obstetric event. Although abruption and retroplacental hemorrhage are sometimes used interchangeably, abruption signifies a clinical event with signs and symptoms, whereas retroplacental hemorrhage is a pathologic finding, often with no clinical correlation (see Chapter 32). Occurring in 1% of pregnancies, abruption is a leading cause of vaginal bleeding in the latter half of pregnancy, and an important cause of perinatal morbidity. It accounts for 12% of perinatal deaths.17 The effect on the mother depends primarily on the severity of the abruption, whereas the effect on the fetus depends both on the severity of the abruption as well as on the fetus’s gestational age. Risk factors include prior abruption, smoking, trauma, multifetal gestation, hypertension, pre-eclampsia, thrombophilias, advanced maternal age, preterm premature rupture of the membranes, intrauterine infections, and polyhydramnios.18 Cocaine abuse may also be a cause. From the pathologist’s viewpoint, chorionic villus hemorrhage and villus edema are more frequent in cocaine users.
Questions frequently posed to the pathologist include: does an abruption exist, how extensive is it, and is it recent or old? More often than not, none of these questions can be answered solely on the pathologic examination, leading the pathologist to come away disappointed at not being able to add much of clinical use. Most are peripheral (Figure 33.3) and, if recent, will not be detected by pathologic examination, since the hemorrhage will have escaped peripherally and presented as vaginal bleeding. Retroplacental hemorrhage toward the center is far more significant (Figure 33.4), since the displaced placenta can no longer support fetal functions. In a study of over 53,000 pregnancies19 the extent of placental separation determined the chance of stillbirth. With a 75% separation, the risk of fetal death was increased 31-fold. Even a 50% separation showed profound effects. The risk for preterm delivery was also substantially increased when the separation was mild (with a separation of 25%, the relative risk was 5). The amount of bleeding is best gauged by the volume of retroplacental clot found at cesarean section, or secondarily by the amount of blood clot that accompanies the placenta to the pathology laboratory. The duration of the bleeding is best gauged by whether the parenchyma has been displaced by the blood, whether the junction of the blood and parenchyma shows discoloration from pigment breakdown, and whether the parenchyma near the abruption is infarcted.
Macroscopically, the location, diameter of the involved area, depth of the crater, and thickness of the normal placenta above should be recorded, as these measurements will help determine the significance of the abruption. Microscopic sections should be taken at several areas of the junction between the blood and placental parenchyma, as the tissue reaction can help date the abruption. Neutrophils in the basal plate above the hemorrhage are the earliest finding, occurring in <4 hours.20 Nearly a day will have passed before the villi degenerate and an inflammatory response appears at the edge of the hemorrhage (Figure 33.5). Within several days there will be evidence of hemoglobin breakdown. Some placentas will show different changes at different areas. Placental abruption is generally regarded as an acute event, but it is often the end result of chronic processes that have begun earlier in pregnancy, even near the time of conception.21 In a cohort of extremely low birth weight infants (<1000 g), placental abruption was found more commonly with histologic chorioamnionitis and funisitis.22
Hypertensive Disorders
Pre-Eclampsia
Pre-eclampsia, a disorder unique to pregnancy, occurs in about 5–8% of pregnant women. Risk factors include nulliparity, women who are at the extremes of reproductive age, multiple gestation, and chronic diseases such as diabetes mellitus, kidney disease, and chronic hypertension. It usually begins insidiously after week 20 of pregnancy with excessive weight gain, marked fluid retention, increase in maternal systemic blood pressure, and the appearance of proteinuria. Pre-eclampsia is diagnosed when the blood pressure is sustained at or above 140 mmHg systolic or 90 mmHg diastolic and the urinary protein excretion exceeds 300 mg/day, but classification systems differ somewhat.23
Pre-eclampsia is classified as early onset (<34 weeks) or late onset. While late onset is by far the more common, it is not associated with placental disease and usually does not result in fetal growth restriction. The points below relate to early onset pre-eclampsia, which has been described as ‘a disease of failed interaction between two genetically different organisms.’24 Along with understanding of the role of implantation has been its description as a two-stage disease.24 Stage 1 is inadequate implantation and stage 2 is the clinical manifestations of endothelial activation in the third trimester. The sequence of events is outlined in Figure 33.6. Decidual natural killer cells, which constitute the majority of decidual white cells in the first trimester, play a key role in trophoblast invasion and successful implantation.25 Persistence of smooth muscle in uterine spiral arteries results in irregular pulsatile flow, with consequent mechanical and oxidative stress to the placenta. This results in increased shedding of trophoblast by necrosis and aponecrosis, which facilitates an inflammatory response in the mother.26 The complex interaction of abnormal placental flow and pre-eclampsia suggests that impaired invasion of deep spiral arteries might result from, rather than cause, maternal flow defects.23
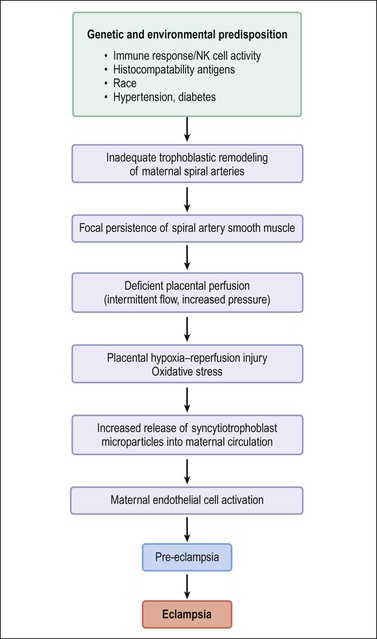
Figure 33.6 Pathogenesis of pre-eclampsia and eclampsia. (Data from Robboy SJ, Duggan M, Kurman RT. The female reproductive system. In: Rubin E, Farber J, editors. Pathology. 3rd ed. Philadelphia: Lippincott; 1999. p. 962–1028.)
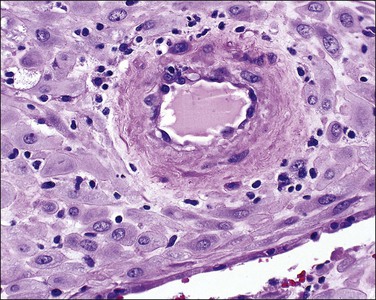
Examination of the placenta from early onset pre-eclampsia should include evaluation of acute and chronic hypoxic changes, with a comment as to the degree of severity. Expected findings include a small placenta with accelerated maturation, ischemic villous crowding, and infarction. Atherosis (Figure 33.7) will be seen in only 50% of cases, but nontransformed vessels (i.e., those whose muscularis has not been replaced by trophoblast and fibrin) (Figures 33.8 and 33.9), and hypertrophic decidual arteriopathy may be present. Laminar decidual necrosis is common. Thrombotic disease in the fetal circulation is three to four times more common when there is uteroplacental ischemic disease, and may be focal or extensive.
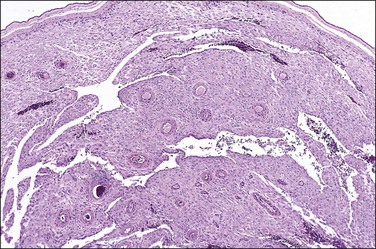
Figure 33.8 Maternal hypertension with a vessel showing atherosis (left) contrasted with untransformed vessels (right).
Intrauterine Growth Restriction
Intrauterine Growth Restriction (IUGR), also known as fetal growth restriction, is where the infant fails to achieve its biologic growth potential. This category overlaps with those who are small for gestational age. Both entities are commonly defined as fetal weight below the 10th percentile for gestational age, but approximately 70% of infants in this group are constitutionally small and have no pathology. Data on race, birth order, and parental body characteristics can help improve identification of true growth restriction.27 Agreement between customized standards and a population standard on growth restriction can be expected in approximately two-thirds of births. Birth weight below the 3rd percentile shows the strongest correlation with perinatal mortality.
IUGR is a late stage manifestation of several disease states. The etiologies can be divided into maternal causes and fetal factors. Chronic maternal hypoxia is an important cause, seen in high altitude, pulmonary hypertension, anemia, hemoglobinopathies, and cardiac failure.28 Fetal factors include fetal genetic disorders, chromosomal abnormalities, and congenital malformations. Mothers who have a history of a prior pregnancy with IUGR are also at risk for recurrence in subsequent pregnancies, including an increased risk of stillbirth.29
Pre-eclampsia is accompanied by IUGR in up to one-third of cases, but IUGR may occur in normotensive pregnancies. Differences in the pathways of spiral artery remodeling may result in the development of IUGR alone, or PET with IUGR.25
Morphologic findings in IUGR in some cases reflect the effects of stress on the placenta. Placental function is compromised by oxidative stress and complement activation, causing a decrease in the functioning trophoblast mass. This is manifest by syncytiotrophoblast apoptosis, syncytial knots, and increased perivillous fibrin.30
Early onset IUGR is less common than late onset. It is characterized by absence or reversal of end-diastolic flow in umbilical artery Doppler studies. Histologic examination shows poor peripheral villous development with nonbranching angiogenesis and increased syncytial knots.31 This cohort with persistent absence or reversal of end-diastolic flow represents a severely affected group in whom early intervention is warranted. Other abnormalities of Doppler flow include abnormalities of the systolic/diastolic ratio and of the pulsatility index. These correlate strongly with placental abnormalities, not just those reflecting maternal underperfusion, but also villous abnormalities and fetal vascular obstruction.32 Epigenetic regulation of the placenta occurs, with imprinted and nonimprinted genes influencing development.33 Overexpression of PHLDA2, a paternally imprinted gene that regulates placental growth, correlates with histologic features of maternal vascular underperfusion.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree