Nodular Sclerosis Hodgkin Lymphoma
C. Cameron Yin, MD, PhD
Key Facts
Terminology
Nodular sclerosis is type of CHL composed of lacunar-type HRS cells and inflammatory cells forming nodules surrounded by fibrous bands
Etiology/Pathogenesis
Hodgkin and Reed-Sternberg (HRS) cells arise from late germinal center B cells
Ig gene rearrangements positive; many defects in B-cell differentiation
Clinical Issues
Represents ˜ 70% of CHL cases in developed countries
15-34 years; mediastinal or cervical lymph nodes
Current chemotherapy ± radiation can cure many patients
Microscopic Pathology
Lymph node architecture effaced by nodules surrounded by broad collagen bands
HRS cells have features of lacunar cells
Background of inflammatory cells
Ancillary Tests
CD30(+) in > 95%; CD15(+) ˜ 70-80% of cases
pax-5(dim +) ˜ 90%, CD20(variably +) ˜ 20%
EBV(+) ˜ 20%, CD45/LCA(-)
Top Differential Diagnoses
Primary mediastinal large B-cell lymphoma
B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and CHL
Lymphocyte-rich classical Hodgkin lymphoma
Nodular lymphocyte predominant HL
TERMINOLOGY
Abbreviations
Nodular sclerosis Hodgkin lymphoma (NSHL)
Synonyms
Nodular sclerosis classical Hodgkin lymphoma
Nodular sclerosis (or sclerosing) Hodgkin disease
Definitions
Classical Hodgkin lymphoma (CHL) is a lymphoid neoplasm composed of Hodgkin and Reed-Sternberg (HRS) cells in a variable inflammatory background
Nodular sclerosis is a type of CHL composed of lacunar-type HRS cells and inflammatory cells forming nodules surrounded by fibrous bands
ETIOLOGY/PATHOGENESIS
Infectious Agents
Epstein-Barr virus (EBV) is present in HRS cells in ˜ 20% of cases and has a probable pathogenetic role
Expression of EBNA1 and latent membrane proteins LMP1 and LMP2a
LMP1 mimics active CD40 receptor
LMP2a mimics B-cell receptor
Pathogenesis
HRS cells arise from late germinal center or early postgerminal center B-cells that
Have undergone immunoglobulin (Ig) gene rearrangements with somatic mutations
Have undergone crippling Ig mutations in subset of cases
Lack B-cell antigen receptors
HRS cells lose much of normal B-cell immunophenotype due to
Severe impairment of transcription factor network regulating B-cell gene expression
Low or undetectable levels of transcription factors: OCT2, BOB1, PU.1, and early B-cell factor (EBF)
Leads to low level of Ig transcripts in HRS cells
Made worse by epigenetic silencing (promoter hypermethylation) of Ig transcription
Impaired function of early B-cell development transcription factors: pax-5, E2A, and EBF
pax-5 and E2A are expressed in HRS cells
Aberrant overexpression of NOTCH1, ABF1, and ID2 inhibit overall B-cell development
Also leads to decreased or absent expression of CD19, CD20, and CD79a
Overall, these abnormalities physiologically should lead to apoptosis
However, HRS cells are rescued from undergoing apoptosis
Development of antiapoptotic mechanisms to achieve survival
Inhibition of executors of apoptosis by expression of X-linked inhibitor of apoptosis (XIAP)
Expression of FLICE-like inhibitory protein
Deregulation of Bcl-2 family proteins
Protection from Fas-induced cell death
Deregulation of signaling pathways
Poorly understood causes
Include paracrine and autocrine feedback loops in addition to genetic lesions of HRS cells
Constitutive activation of NF-κB pathway: Canonical and alternative pathways
Activation of Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway
Role of microenvironment
Reactive cellular infiltrate is induced, in part, by HRS cells
Protects HRS cells from apoptosis
Suppresses T-cell and NK-cell immune response against HRS cells
HRS cells produce a variety of molecules
Th2 cytokines, chemokines, growth factors, and their receptors
IL-1, IL-10, TNF-α, TGF-β, and eotaxin
Most cytokines signal via JAK/STAT pathway
HRS cells in NSHL show increased production of IL-13
May be responsible for broad bands of birefringent collagen
Possible Origin
Thymic B cell in patients with mediastinal involvement
CLINICAL ISSUES
Epidemiology
Incidence
Represents ˜ 70% of CHL cases in developed countries
Relatively less frequent in underdeveloped nations
Age
Peak at 15-34 years
Gender
Slightly more prevalent in women
Ethnicity
More common in whites than in African-Americans or Latino-Americans
Site
Mediastinal or cervical lymph nodes
Presentation
Mediastinal involvement in ˜ 80% of cases
Bulky disease in ˜ 50% of cases
B symptoms in ˜ 40% of cases
Associated predominantly with clinical stage II
Treatment
Current chemotherapy &/or radiation can cure disease in many patients
Chemotherapy with or without radiation
ABVD: Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine
Chemotherapy alone or reduced cycles for early stage NSHL
Prognosis
> 90% survival at 5 years in patients with early stage disease
Therapy modifies prognosis
Adverse prognostic factors
Advanced stage
Massive mediastinal involvement
Older age, usually > 45 years
Male gender
Histologic grading of NSHL is predictive but less important than clinical factors
Recurrent disease with multiple adverse factors results in 56% overall survival at 5 years
Deaths mostly related to 2nd malignancy, therapeutic toxicity, and older age
IMAGE FINDINGS
General Features
Current imaging techniques have made staging laparotomy obsolete
MACROSCOPIC FEATURES
General Features
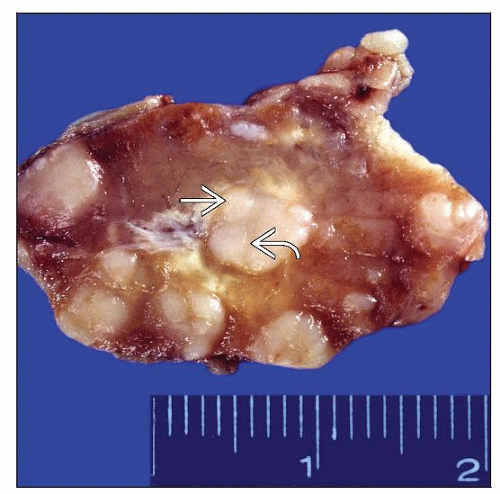
Nodular surface; nodules often surrounded by bands of fibrosis
MICROSCOPIC PATHOLOGY
Histologic Features
Lymph node architecture effaced by neoplastic nodules surrounded by broad collagen bands
Originate in thickened capsule
Dissect lymph node into nodules of various sizes
Lacunar cells formed due to retraction artifact of HRS cells in formalin-fixed tissue sections
Nuclei tend to be lobated with smaller lobes, less prominent nucleoli than HRS of other CHL types
Number of HRS cells and lacunar cells highly variable
Lacunar cells may form cell aggregates associated with necrosis and histiocytes
Background of inflammatory cells
Eosinophils, histiocytes, &/or neutrophils are often numerous
Occasional eosinophilic abscesses are noted
Cytologic Features
Lacunar cells in an inflammatory background can be appreciated in fine needle aspiration smears
Immunophenotype can be assessed in cell block
Syncytial Variant of NSHL
Confluent aggregates of lacunar cells
Cohesive appearance may resemble large cell non-Hodgkin lymphoma or metastatic carcinoma
Limited number of birefringent collagen bands and occasional necrosis
Grading of NSHL
British National Lymphoma Investigation (BNLI) system developed in 1989
Based on amount of HRS cells, anaplasia of HRS cells, and fibrosis features
Grade 1 NS: Scattered HRS cells in lymphocyte-rich or mixed cellular infiltrate
Grade 2 NS: Aggregates of HRS cells or pleomorphic cytology in > 25% of nodules
Grade showed differences in outcome for patients with advanced disease only
Lack of prognostic significance with current chemotherapeutic regimens
German Hodgkin Lymphoma Study Group system reported in 2003
Similar to BNLI system but also includes tissue eosinophilia (> 5% of cell infiltrate)
Controversial results; prognostic value for intermediate and high-stage disease
Extranodal Involvement of NSHL
Spleen
˜ 30% of patients with NSHL
Usually presents as solitary or multiple nodules
Tumor nodules surrounded by sclerosis that effaces splenic architecture
Incipient lesions are periarteriolar or at periphery of marginal zone
Nodules of NSHL in spleen do not necessarily show fibrous bands
Liver
˜ 10% of patients with NSHL; usually microscopic clusters
Mainly detected in wedge biopsies of staging laparotomy (procedure now obsolete)
Infiltrates with preferential portal or portal to central vein distribution
Associated with constitutional symptoms and biochemical abnormalities
Sometimes nondiagnostic inflammatory changes, without HRS cells
Bone marrow (BM)
˜ 5-10% of cases of NSHL, up to 70% in necropsies
Can be detected during staging of CHL or may be presenting finding
CHL presenting in BM usually manifests with cytopenias
Unlikely involvement in young patients with normal blood counts and low-stage disease
Likely involvement in older patients with cytopenias, B symptoms, and high-stage disease
Variable extent of involvement, amount of neoplastic cells, and stromal changes
Eosinophilia may be prominent including microabscesses
Diffuse stromal fibrosis and histiocytic infiltrate may obscure HRS cells
Thymus
NSHL is type of CHL most frequently associated with mediastinal involvement
Thymus is commonly involved and may be cystic
In some cases, granulomatous inflammation can obscure neoplastic cells
ANCILLARY TESTS
Immunohistochemistry
CD30(+) in > 95%; CD15(+) in 70-80% of cases
Characteristic membranous pattern with accentuation in Golgi area
pax-5(dim +) ˜ 90%, CD20(variably +) ˜ 20%, CD79a(+) ˜ 10-20%
Ki-67(+), p53(+), MUM1(+)
CCL17(+), Fascin(+/-), Bcl-2(+/-)
CD45/LCA(-), EMA(-), Ig(-), clusterin(-)
OCT2(-/+), BOB.1(-/+), PU.1(-)
EBV(+) with latency type II pattern in ˜ 20% of cases
LMP-1(+), LMP2a(+), EBNA1(+), EBNA2(-)
T-cell antigens can be aberrantly expressed by HRS cells in up to 15% of cases
Background CD4(+) T cells form rosettes around HRS cells
Flow Cytometry
Polytypic B cells and T cells with normal immunophenotype, CD4:CD8 ratio often elevated
Useful to exclude non-Hodgkin lymphoma
Cytogenetics
Data derived from HL cell lines and primary HRS cells
Aneuploidy and hypertetraploidy
Random numerical chromosomal aberrations
In Situ Hybridization
EBER(+) in ˜ 20% of cases
Array CGH
Molecular Genetics
Monoclonal Ig gene rearrangements of HRS cells
Rearranged Ig genes harbor somatic mutations in IgH variable regions
Rare (˜ 2%) cases reported with monoclonal T-cell receptor gene rearrangements
Unclear if these cases are truly examples of CHL
REL gene on 2p16 that encodes 1 component of NF-κB shows gains or amplifications in 50% of CHL
Inactivating mutations of NF-κB inhibitor IκBα in 10-20% of CHL
Gene Expression Profiling
Signature of NSHL shares features with primary mediastinal large B-cell lymphoma
DIFFERENTIAL DIAGNOSIS
Primary Mediastinal Large B-cell Lymphoma (PMBL)
Nodal and soft tissue effacement
Interstitial collagen deposition surrounding clusters or sheets of large lymphoma cells
Large cells often exhibit cytoplasmic retraction artifact
Immunophenotype of neoplastic B cells
CD19(+), CD20(+), CD22(+), CD45/LCA(+), CD79a(+)
CD30(+/-) and often dim; MAL(+/-)
Surface Ig(-), CD10(-), CD15(-)
B-cell Lymphoma, Unclassifiable, with Features Intermediate Between DLBCL and CHL
a.k.a. “gray zone lymphoma”
Mostly located in mediastinum; men 20-40 years of age
Morphologic &/or immunophenotypic overlap between DLBCL (often PMBL) and CHL
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree