Nodular Lymphocyte Predominant Hodgkin Lymphoma
Pei Lin, MD
Key Facts
Clinical Issues
Peak incidence in 4th decade, affects all age groups
Patients often present with stage I or II nodal disease
Cervical, axillary, or inguinal lymph nodes
Slow progression and frequent relapses
Excellent prognosis
˜ 3-5% of patients develop large B-cell lymphoma
Microscopic Pathology
Nodular or nodular and diffuse patterns
LP cells scattered in background of small lymphocytes and histiocytes
LP cells have vesicular chromatin, inconspicuous nucleolus, and thin nuclear membrane
Mixture of different cell types gives “moth-eaten” pattern on low-power view
No necrosis or thick fibrous bands
Uninvolved lymph node shows reactive follicular hyperplasia &/or PTGC
Ancillary Tests
Immunophenotype of LP cells
CD20(+), CD22(+), CD45/LCA(+), CD79a(+)
Bcl-6(+), pax-5(+), OCT2(+), BOB1(+)
CD15(-), CD30(-), EBV(-), Bcl-2(-)
Single-cell PCR
LP cells carry monoclonal IgH gene rearrangements
Top Differential Diagnoses
Lymphocyte-rich classical Hodgkin lymphoma
T-cell/histiocyte-rich large B-cell lymphoma
Progressive transformation of germinal centers
Follicular lymphoma
Nodular sclerosis Hodgkin lymphoma
TERMINOLOGY
Abbreviations
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL)
Synonyms
Nodular lymphocyte predominant Hodgkin disease (REAL, 1994)
Lymphocytic-predominant Hodgkin disease (Rye, 1966)
Lymphocytic &/or histiocytic predominance Hodgkin disease (Lukes and Butler, 1966)
Paragranuloma (Jackson and Parker, 1944)
Definitions
Nodular proliferation of scattered large neoplastic B cells associated with numerous inflammatory cells
Neoplastic cells are designated as lymphocyte-predominant (LP) cells
a.k.a. “popcorn” cells because of their hyperlobated nuclei with vesicular chromatin
Formerly called L&H (lymphocytic &/or histiocytic) cells
Neoplastic cells are usually confined within follicular dendritic cell meshworks
Background infiltrate of nonneoplastic small lymphocytes and histiocytes
Inflammatory cells greatly outnumber neoplastic LP cells
Diffuse form of NLPHL
Term derived from Lukes and Butler classification, but its existence has been challenged
Most cases in this category have been reclassified as
T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL)
NLPHL with diffuse THRLBCL-like areas
Classical Hodgkin lymphoma (CHL)
Rare cases of diffuse NLPHL probably exist, i.e., “moth-eaten” B-cell-rich pattern of NLPHL
ETIOLOGY/PATHOGENESIS
Postulated Normal Counterpart
Germinal center B lymphocyte at centroblast stage of differentiation
Associated Lesions
NLPHL is associated with progressive transformation of germinal centers (PTGC)
PTGC and NLPHL can involve same lymph node biopsy specimen
In past, PTGC often identified in staging laparotomy specimens of NLPHL patients
However, prospective studies of patients with PTGC show no increased risk of NLPHL
CLINICAL ISSUES
Epidemiology
Incidence
5-6% of all Hodgkin lymphomas
Age
Median: 35 years
All age groups are affected
Gender
Male predominance
Male to female ratio > 2:1
Site
Lymph nodes
Most commonly affected groups include cervical, axillary, or inguinal lymph nodes
Paraaortic and iliac lymph nodes less often involved
Liver &/or spleen involved in ˜ 10% of cases
Mediastinum involved in ˜ 7%
Bone marrow rarely involved (˜ 2%)
Bone marrow involvement is usually evidence of transformation to large B-cell lymphoma
Presentation
Peripheral lymphadenopathy
Stage I or II in ˜ 80% of patients
B symptoms are uncommon (˜ 10%)
Laboratory Tests
Normal complete blood count; no leukemic phase
Serum lactate dehydrogenase (LDH) or β-2-microglobulin levels are rarely elevated
Natural History
NLPHL is clinically indolent disease with frequent relapses
Relapse-free survival curves show “staircase”
No plateau suggestive of cure
Early and late (> 10 years) relapses occur
Risk of relapse is independent of stage of disease or therapy
Relapse can be localized or generalized (˜ 20%) disease
˜ 3-5% of NLPHL transform to large B-cell lymphoma (LBCL)
Large B-cell lymphoma typically follows NLPHL but can coexist with or precede NLPHL
Subset of transformed cases resembles diffuse large B-cell lymphoma (DLBCL)
Clinically indolent when compared with de novo diffuse large B-cell lymphoma
2nd subset resembles THRLBCL
˜ 15% of patients die of disease with prolonged followup
Deaths related to therapy-refractory disease or 2nd malignancies
2nd malignancies represent ˜ 4% of all deaths
Acute leukemia (2%), non-Hodgkin lymphoma (1%), solid organ tumors (1%)
Treatment
Options, risks, complications
“Watch and wait” has been advocated for pediatric patients with localized disease
Drugs
Combination chemotherapy employed most often
Recommended regime: Doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD)
Rituximab (anti-CD20) monoclonal antibody is often used
As part of ABVD regimen, upfront (R-ABVD)
At time of refractory disease
Radiation
Localized disease may be treated with involved field radiation alone
This option is avoided in pediatric and adolescent patients to avoid injuring growth plates of bones
Prognosis
Good prognosis with > 80% 10-year survival
Better survival for patients with low- vs. high-stage disease
Patients with NLPHL have better survival than patients with classical Hodgkin lymphoma
Transformation to diffuse large B-cell lymphoma or THRLBCL is often associated with poorer prognosis
Bone marrow involvement is associated with aggressive clinical behavior
Prognosis may not be impacted if large B-cell lymphoma is localized and treated appropriately
IMAGE FINDINGS
Radiographic Findings
Peripheral lymphadenopathy
NLPHL lesions are not FDG-PET avid
MICROSCOPIC PATHOLOGY
Histologic Features
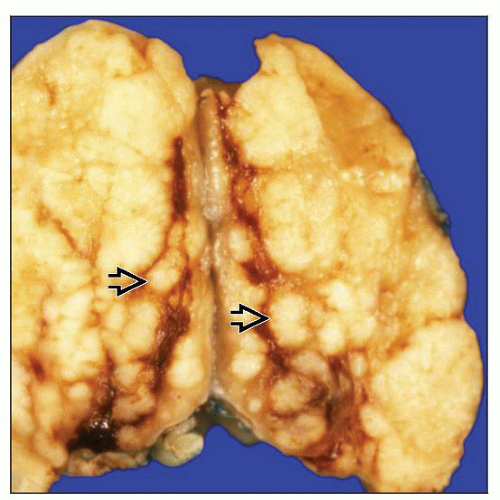
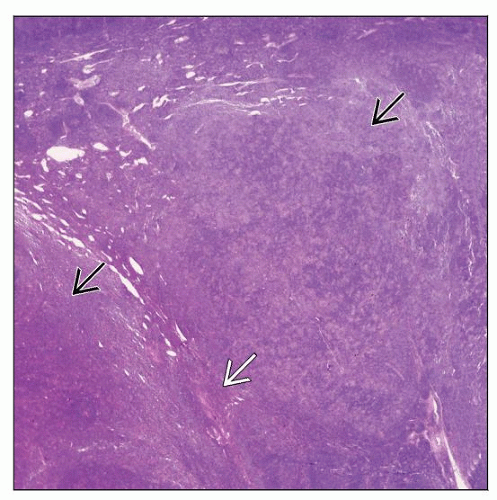
Complete or partial effacement of lymph node architecture
Nodular or nodular and diffuse patterns
Expansile nodules composed mostly of small lymphocytes and fewer histiocytes
Reactive lymphoid follicles usually absent within nodules
Absent or rare centrocytes or centroblasts within nodules
Nodules larger than normal lymphoid follicles
LP cells are large and scattered amongst abundant small lymphocytes and histiocytes
Represent ˜ 1% of all cells
LP cells have variety of appearances
Multilobated “popcorn” cells with vesicular chromatin and multiple small nucleoli
Multinucleated or mummified cells
LP cells also can be round without multilobation
Various architectural patterns have been described
Classical nodular pattern is most common
Serpiginous nodular pattern
Confluent irregular nodules
Nodular with extranodular LP cells
Pattern more commonly seen in patients with recurrence
Nodular pattern with T-cell-rich background
THRLBCL-like
Always associated with at least 1 typical nodule of NLPHL
Diffuse areas indistinguishable from primary THRLBCL
Most background lymphocytes are T cells and histiocytes
Absence of underlying follicular dendritic cell meshworks
Associated with B symptoms and higher clinical stage
Diffuse, B cell-rich with “moth-eaten” appearance
Uncommon pattern (< 5% of cases)
Most background lymphocytes are B cells
Underlying follicular dendritic cell meshworks positive
Histiocytes may be epithelioid &/or form small granulomas
Features common in classical Hodgkin lymphoma are usually absent in NLPHL
Eosinophils, neutrophils, and plasma cells are unusual
Classical Hodgkin and Reed-Sternberg (HRS) cells are absent or rare
Necrosis is rare; no fibrous bands around nodules
Residual/uninvolved lymph node in biopsy specimens of NLPHL
Reactive follicular hyperplasia is usually present
PTGC commonly present
Recurrent/relapsed NLPHL
Depletion of small lymphocytes with increased histiocytes
Fibrosis in up to 40% of cases with recurrence
Diffuse areas present; often increased in size
Cytologic Features
Diagnosis of NLPHL difficult to establish in fine needle aspirate specimens
Nodular architecture difficult to appreciate in smears
Small lymphocytes, histiocytes, and large LP cells present
No granulocytes or plasma cells
Transformation of NLPHL to Large Cell Lymphoma
Large cell lymphoma may coexist with or follow NLPHL
Large cells may form sheets, as in de novo diffuse large B-cell lymphoma, or be scattered, as in THRLBCL
No consensus on pathologic criteria to distinguish between
NLPHL with diffuse THRLBCL-like areas vs. transformation to THRLBCL
Transformation of NLPHL to THRLBCL can be diagnosed when
Diffuse areas of THRLBCL are identified, and
Patients have high-stage disease, including bone marrow involvement, &/or other clinical evidence of transformation, such as
High serum lactate dehydrogenase or β-2-microglobulin levels
Lytic bone lesions
Bone marrow involvement in patients with NLPHL is usually evidence of transformation
Extensive liver involvement is usually associated with transformation
Transformation of NLPHL to diffuse large B-cell lymphoma
Sheets of large neoplastic cells outside nodules of NLPHL
ANCILLARY TESTS
Immunohistochemistry
LP cells
CD20(+), CD22(+), CD79a(+), CD75(+)
pax-5(+), OCT2(+), BOB1(+), PU.1(+)
CD40(+), CD80(+), CD86(+)
Bcl-6(+), AID(+), SWAP-70(+)
CD45/LCA(+), Ki-67 (proliferation) high
EMA and MUM1(+) in ˜ 50% of cases
IgD(+) in ˜ 25% of cases
IgD correlates with younger patient age
Pan-T-cell antigens(-), Bcl-2(-)
CD15(-) and CD30(-)
CD30(+) LP cells reported in ˜ 10% of NLPHL cases
CD30(+) reactive immunoblasts are common in NLPHL
CD15(+) LP cells very rare; often at time of relapse
Epstein-Barr virus (EBV)-LMP1(-)
Rare (< 1%) cases of NLPHL with EBV(+) LP cells reported in developed countries
Background inflammatory infiltrate
Small lymphocytes are mixture of B and T cells
B cells
T cells
CD2(+), CD3(+), CD5(+), CD7(+)
Form “rosettes” around LP cells
Minor population of CD3(+) cells is of follicular Thelper cell lineage
CD3(+), CD4(+), CD57(+)
CD10(+), Bcl-6(+) < PD-1(+)
Form “rosettes” around LP cells in ˜ 50% of cases
Follicular dendritic cell meshworks are present in nodular areas
CD21(+), CD23(+), &/or CD35(+)
Histiocytes
CD68(+)
Recurrent/relapsed NLPHL
Depletion of background small B cells
Decreased or absent follicular dendritic cells
Increased numbers of background T cells and histiocytes
LP cells may express CD30 or rarely CD15
Flow Cytometry
Polytypic B cells
Mature T cells
CD4(+), CD8(+) T cells in ˜ 50% of cases
Large neoplastic cells are lost or overlooked in routine flow cytometric analysis
Cytogenetics
Usually complex structural karyotypic aberrations
Chromosome 3q27 (BCL6 locus) involved in up to 60% of cases
In Situ Hybridization
EBER(-) in LP cells
< 1% of NLPHL cases are EBER(+) in Western countries
EBV may be more common in LP cells of NLPHL in developing countries
PCR
Monoclonal IgH or light chain gene rearrangements when using single-cell PCR analysis
Rearrangements often not detectable using standard PCR or Southern blot methods and whole biopsy specimens
Array CGH
30-60% of cases may show gains or losses of chromosomes
Gains: Chromosomes 1, 2q, 3, 4q, 5q, 6, 8q, 11q, 12q, and X
Loss: Chromosome 17
Molecular Genetics
Frequent somatic mutations of IgH variable region
Evidence of ongoing mutations
BCL6 gene rearrangements in ˜ 50% of cases
IgH is most common partner
Other partners: Chromosome loci 2q23; 5q31, 6q22, 9q22, and 17p21
DIFFERENTIAL DIAGNOSIS
Lymphocyte-rich Classical Hodgkin Lymphoma, Nodular Variant
Form of classical Hodgkin lymphoma with prominent nodular pattern that can closely mimic NLPHL
Nodules composed of prominent mantle zones with atrophic or absent germinal centers
HRS cells in mantle zones of enlarged lymphoid follicles
Immunophenotype
HRS cells are CD15(+), CD30(+), EBV-LMP1(+/-), CD45/LCA(-)
T-cell/Histiocyte-rich Large B-cell (THRLBCL) Lymphoma
Affects elderly patients; rare in children and adolescents
B symptoms, high stage, and elevated serum LDH levels
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree