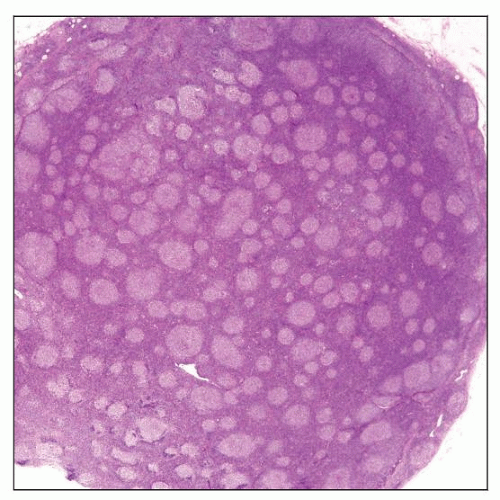
Nodal Follicular Lymphoma
C. Cameron Yin, MD, PhD
Key Facts
Terminology
B-cell neoplasm composed of germinal center B cells (centrocytes and centroblasts)
Etiology/Pathogenesis
Overexpression of antiapoptotic Bcl-2 due to t(14;18) (q32;q21)
Susceptibility locus at 6p21.3 and higher risk in 1st-degree relatives of patients with FL
Clinical Issues
˜ 20% of NHL, 2nd overall, in USA and Western Europe
Usually asymptomatic although disseminated at presentation
Overall 10-year survival is up to ˜ 80%
Microscopic Pathology
Closely packed neoplastic follicles, fairly uniform in size and shape
Neoplastic follicles composed of variable amounts of centrocytes and large centroblasts
Grading has prognostic and therapeutic significance
Ancillary Tests
B cells positive for Bcl-2, Bcl-6, and CD10
Bcl-2(+) in 85-90% of FL grade 1 and grade 2; 50% in FL grade 3
Top Differential Diagnoses
Reactive follicular hyperplasia
Nodular lymphocyte predominant HL
Mantle cell lymphoma
Nodal marginal zone lymphoma
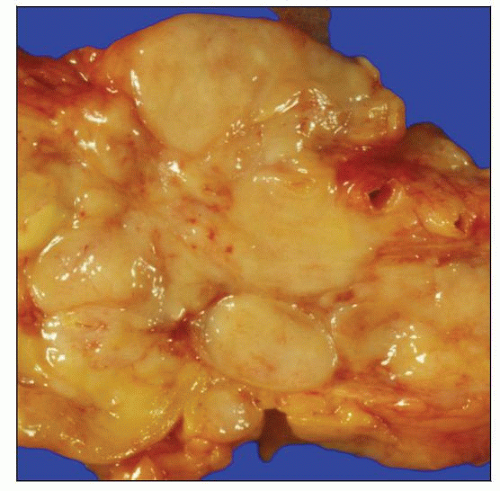 Gross photograph shows matted mesenteric lymph nodes involved by low-grade follicular lymphoma (FL). This specimen was obtained at time of autopsy. |
TERMINOLOGY
Abbreviations
Follicular lymphoma (FL)
Synonyms
Follicle (germinal) center cell lymphoma
Centroblastic/centrocytic lymphoma
Definitions
B-cell neoplasm composed of germinal center B cells (centrocytes and centroblasts)
Follicular, follicular and diffuse, and diffuse growth patterns
ETIOLOGY/PATHOGENESIS
t(14;18)(q32;q21) Resulting in Overexpression of Bcl-2
Bcl-2 is antiapoptotic and confers survival advantage
t(14;18) is considered initiating molecular event of FL
Insufficient to induce lymphomagenesis by itself
Other molecular changes necessary for development of lymphoma
Imbalance of Other Proteins Involved in Apoptosis
Overexpression of cell death suppressor proteins BCL-xL and MCL1
Decreased expression of cell death promoting proteins BAX and BAD
Overexpression of inhibitors of apoptosis proteins (IAP)
Germline Susceptibility Factors
Genotypic analysis has identified novel susceptibility locus at 6p21.3
Contains single gene, chromosome 6 open reading frame 15 (C6orf15)
4-fold increased lymphoma risk in 1st-degree relatives of patients with FL
Association of single nucleotide polymorphisms of estrogen receptor gene with reduced risk of FL
Immunologic Microenvironment
CD40L(+) T cells in secondary follicles inhibit FL cell death
Follicular dendritic cells contribute to preventing apoptosis of FL cells
CLINICAL ISSUES
Epidemiology
Incidence
˜ 20% of NHL; 2nd most common NHL in USA and Europe
Uncommon in Asia and underdeveloped countries
Age
Median = 59 years
Gender
M:F = 1:1.7
Site
Cervical and inguinal lymph nodes are more frequently affected
Commonly affected extranodal sites
Bone marrow, spleen, liver, and peripheral blood
FL uncommonly arises at extranodal sites
Skin, gastrointestinal tract, thyroid gland, testis
Presentation
Insidious onset
Often asymptomatic at time of initial diagnosis
Almost always disseminated (stages III-IV)
Natural History
Indolent clinical course but frequent relapses
Some cases progress to diffuse large B-cell lymphoma (DLBCL)
Treatment
In the past, “watch and wait” strategy was usually employed for asymptomatic patients
Chemotherapy is currently used upfront more often for patients with stage III-IV disease
Rituximab, cyclophosphamide, adriamycin (doxorubicin), vincristine, and prednisone (R-CHOP)
Bulky disease or signs of progression necessitate chemotherapy
Radiation has value for subset of patients with stage I and II disease
Prognosis
Overall 10-year survival is up to ˜ 80%
Adverse prognostic factors summarized in FL International Prognostic Index 2 (FLIPI 2)
High serum β2-microglobulin
Bulky lymph nodes > 6 cm
Bone marrow involvement
Hemoglobin < 12 g/dL
Age > 60 years
FLIPI 2 prognostic model stratifies patients into different prognostic risk groups
Model developed in post rituximab era using prospective analysis
Pathologic adverse prognostic factors include
High histologic grade and diffuse areas > 25% with predominance of large cells
These areas are designated as DLBCL
High proliferation index
Complex karyotype
Del6q23-26; del17p and mutation of TP53
IMAGE FINDINGS
General Features
Widespread lymphadenopathy; often small lymph nodes
MACROSCOPIC FEATURES
General Features
Replacement of nodal parenchyma by “fish-flesh” tumor; ± nodularity
MICROSCOPIC PATHOLOGY
Histologic Features
Lymph node
Partial or complete effacement of architecture
Closely packed neoplastic follicles, fairly uniform in size and shape
Follicles usually poorly circumscribed with faint or absent mantle zones
“Cracking” artifact may surround neoplastic follicles
Neoplastic follicles are composed of centrocytes and centroblasts
Cells randomly distributed throughout individual follicles, without polarity
Infrequent mitoses and absent or scanty tingible body macrophages
Centrocytes: Small to large with angulated, elongated, or twisted nuclei, with dark chromatin and scant cytoplasm
Centroblasts: Large cells with oval or multilobated nuclei, vesicular chromatin, 1-3 nucleoli, and moderate cytoplasm
Diffuse areas with or without sclerosis
More frequent in mesenteric and retroperitoneal lymph nodes
Scattered interfollicular neoplastic lymphocytes are not considered as diffuse growth pattern
Follicular dendritic cell meshworks are absent in diffuse areas
Bone marrow
Paratrabecular aggregates of centrocytes and, less frequently, centroblasts in bone marrow
Aspirate smears may have scant lymphoma cells or are negative
Interstitial &/or diffuse patterns in advanced disease
Peripheral blood
Marked leukemic involvement in 5-10% of patients
Neoplastic cells have highly cleaved nuclei and are known as “buttock cells”
Low-level involvement is detected by molecular methods in ˜ 90% of patients
Liver
Portal tracts are preferentially involved
Large mass lesions usually indicate transformation to DLBCL
Spleen
Preferential involvement of white pulp
Unusual morphologic variants of FL
Floral variant
Mantle zone lymphocytes penetrate into neoplastic follicles, imparting irregular shapes
Better highlighted with follicular dendritic cell markers, e.g., CD21
Often grade 3
Plasmacytic differentiation
Focal plasmacytic differentiation can occur rarely in FL, intrafollicular or interfollicular
Extreme degrees with intracytoplasmic inclusions appear as “signet ring cells”
Marginal zone differentiation
Monocytoid cells with clear cytoplasm at periphery of neoplastic follicles
Has been correlated with poorer prognosis
Cytologic Features
Diagnosis of FL can be established by FNA with ancillary support
In smears, aggregates of cells bound by follicular dendritic cells
Variable mixture of centrocytes and centroblasts
Usually, absence of tingible body macrophages
Grading of FL
Grading has prognostic and therapeutic significance
Most reliably performed on lymph node biopsy specimen
System is based on mean number of centroblasts per high power field (HPF)
Count 10 HPFs and divide by 10
Grade 1: 0-5 centroblasts/HPF
Grade 2: 6-15 centroblasts/HPF
Grade 3: > 15 centroblasts/HPF
Grade 3A: Centrocytes admixed with centroblasts
Grade 3B: Sheets of centroblasts with rare or no centrocytes
Remember: Cutoff values are based on 40x objective and 18 mm field-of-view ocular
Many microscopes have larger field-of-view ocular
20 mm field-of-view ocular: Divide 10 HPF count by 12
22 mm field-of-view ocular: Divide 10 HPF count by 15
2008 WHO classification recommends lumping cases of FL 1-2 together as low grade
Minimal differences in outcome between patients with FL grade 1 vs. 2
Diffuse areas > 25% of grade 3 FL should be diagnosed as DLBCL
Histologic Discordance (Discrepant Histology) in Patients with FL
FL involving different lymph node groups may show different grades
Occurs in up to 1/3 of patients who undergo staging laparotomy
Lymph node can be involved by grade 3 FL or DLBCL with bone marrow showing grade 1 FL
Occurs in ˜ 10-20% of patients with grade 3 FL or DLBCL
Low-grade bone marrow involvement does not affect prognosis
Reporting Pattern in FL
Most reliably performed on lymph node biopsy specimen
Follicular: > 75% follicular
Follicular and diffuse: 25-75% follicular
Focally follicular: 1-25% follicular
Diffuse: 0% follicular
Diffuse Follicular Lymphoma
Diffuse growth of small centrocytes with few or absent centroblasts
Immunophenotype: CD10(+), Bcl-6(+), Bcl-2(+)
IgH-BCL2 fusion gene or t(14;18)(q32;q21) present
Rare diagnosis; more common in core needle biopsy specimens
Extensive sampling may reveal focal follicular pattern
Intrafollicular Neoplasia/In Situ Follicular Lymphoma
Lymph node with widely spaced follicles of which a subset have Bcl-2(+) germinal centers
Bcl-2 expression by germinal centers is characteristically bright
Bcl-2(+) follicles have immunophenotype of FL and t(14;18)
Using histologic criteria alone, diagnosis of FL can be difficult or not possible
Patients with intrafollicular neoplasia may
Have FL elsewhere simultaneously or develop FL subsequently
Have other types of non-Hodgkin lymphoma or Hodgkin lymphoma simultaneously or subsequently
Not develop lymphoma on clinical follow-up
Clinically Aggressive B-cell Lymphoma
FL transformation to more clinically aggressive B-cell lymphomas occurs in ˜ 30% of FL patients
Usually transforms into DLBCL
Accounts for most disease-related deaths
Transformed tumor less often resembles Burkitt lymphoma (BL) or tumor with features intermediate between BL and DLBCL
Transformation is commonly associated with
Resistance to therapy and median survival ˜ 1 year
Inactivation of P53 or P16; activation of MYC
Pediatric FL
Localized disease, usually involves neck lymph nodes
Extranodal sites also affected: testis, Waldeyer ring
High histological grade; usually with large follicles
Usually Bcl-2(-) and lacks t(14;18)(q32;q21) or IgH-BCL2
Most patients have good prognosis without disease progression
ANCILLARY TESTS
Immunohistochemistry
Monotypic surface Ig(+); pan-B-cell markers(+)
CD10(+), Bcl-6(+)
CD10 and Bcl-6 more brightly expressed within follicles than in interfollicular regions
HGAL(+), LMO2(+)
Bcl-2(+) in 85-90% of FL grade 1 and grade 2; 50% in FL grade 3
Bcl-2(+) is useful to distinguish FL from reactive follicles that are Bcl-2(-)
Follicular dendritic cell meshworks are present in follicles
Variable expression of CD21, CD23, or CD35
CD23(+/-), IRF-4/MUM1(-)
FLs are usually CD5(-), CD43(-)
Small subset (< 5%) can be CD5(+) or CD43(+)
CD2(-), CD3(-), CD4(-), CD7(-), CD8(-)
Proliferation rate of FLs assessed by Ki-67
Percentage of Ki-67(+) cells correlates with grade
Most low-grade FLs show low proliferation rate (< 20%)
High-grade FLs show moderate to high proliferation rate (> 40%)
Approximately 20% of low-grade FLs have moderate/high proliferation rate
These FLs appear to behave more aggressively, similar to grade 3A FL
Grade 3 FLs
Can be CD10(-), Bcl-2(-), IRF-4/MUM1(+)
Cytogenetics
80-90% of cases have t(14;18)(q32;q21)
Juxtaposes BCL2 at 18q21 adjacent to IgH on derivative chromosome 14
Is rarely (10%) the only karyotypic abnormality
Other common chromosomal aberrations in FL include
Deletions of 1p, 6q, 10q, 17p
Gains of 1, 6p, 7, 8, 12q, 18q, X
Complex karyotype correlates with poorer prognosis
In Situ Hybridization
FISH can detect t(14;18)(q32;q21) in up to 90% of FL cases
Large probes can detect multiple breakpoints
PCR
Monoclonal IgH and Ig light chain gene rearrangements
Variable regions of Ig genes undergo extensive and ongoing mutations
Mutations can cause false-negative result when using PCR to assess for Ig gene rearrangements
Multiple primer sets are therefore required for analysis
There are multiple breakpoints in Bcl-2 that must be individually assessed by PCR
Major breakpoint cluster region (MBR): ˜ 50-60% of FLs with t(14;18)
Minor breakpoint cluster region (MCR): ˜ 5-10% of FLs
Intermediate cluster region (ICR): ˜ 10-15% of FLs
5′ breakpoint region: ˜ 5% of FLs
Array CGH
˜ 90% of FLs have abnormalities detected by CGH or array CGH
Gains: 2p15, 7p, 7q, 8q, 12q, 18p, 18q
Losses: 1p36, 3q, 6q, 9p, 11q, 13q, 17p
Abnormalities associated with worse prognosis
Loss of 6q or 9p21
Gain of chromosome X
Abnormalities associated with transformation to DLBCL
Gains of 2, 3q, and 5