Niacin1
James B. Kirkland
1Abbreviations: ACMS, 2-amino-3-carboxymuconic-6-semialdehyde; ACMSD, 2-amino-3-carboxymuconic-6-semialdehyde decarboxylase; ADP, adenosine diphosphate; ART, mono-ADP-ribosyltransferase; ATP, adenosine triphosphate; BER, base excision repair; CICR, calcium-induced calcium release; DRI, dietary reference intake; GI, gastrointestinal; GRP, glucose-regulated-protein; IP3, inositol triphosphate; NAADP, nicotinic acid adenine dinucleotide phosphate; NAD, nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NER, nucleotide excision repair; NFκB, nuclear factor κB; PARP, poly(ADP-ribose) polymerase; RDA, recommended dietary allowance; TCA, tricarboxylic acid; UL, tolerable upper intake level.
HISTORICAL OVERVIEW
Pellagra is the clinical disease of niacin deficiency in humans. It is mainly caused by dependence on corn as a staple food. Although pellagra likely occurred at a low incidence throughout history, it reached epidemic proportions in the southern United States and Europe as corn-based agriculture spread (1). The term pellagra was derived from the Italian name for the condition, meaning “rough skin.” Corn does contain niacin, but in tightly bound structures; this binding is heat stable, although it is sensitive to alkaline treatment (2). Native Americans had developed various alkaline processing techniques to release the existing niacin, and the importance of this processing was not recognized when Columbus brought corn back to Europe (1).
Pellagra is characterized by the three “Ds” of dermatitis (sun sensitive), dementia, and diarrhea. Diarrhea is the least unique of these, but it leads to a vicious cycle of worsening status of niacin and other nutrients. Anorexia also tends to set in as the deficiency progresses, usually leading up to the death of the patient. The development of the more unique signs of dermatitis and dementia can be unpredictable from patient to patient, thus making diagnosis of pellagra difficult in many cases. The epidemic in the southern United States occurred largely in people working outdoors, and sun-induced skin lesions were a clinical focus (3). Similar outbreaks occurred in Spain, Italy, and Egypt during the 1700s and 1800s. The reported incidence in northern Europe was much lower (3), but cooler weather and indoor work environments may have caused pellagra to manifest as poorly diagnosed dementia, for which unfortunate patients were often confined to an asylum and fed a disease-perpetuating corn-based diet. Even in the southern United States in the 1900s, outbreaks of pellagra were described in asylum populations (3). Women were much more likely to develop pellagra than men, possibly because of an unequal division of food resources (1).
Remarkably, it took several hundred years for the dietary reliance on corn to be accepted as the cause of pellagra, although corn consumption was originally proposed to carry a disease or toxin. Starting in 1915, Dr. Joseph Goldberger conducted clinical trials in which pellagra was induced in prison populations and was cured or prevented by balanced diets or yeast supplements (4). Although nicotinic acid was first isolated in 1867, its role as the active vitamin was not identified until 1937, when black tongue in dogs was used as an animal model of pellagra (5). A host of publications in 1937 to 1938 demonstrated that nicotinic acid cured pellagra in humans (3), and Drs. Douglas Spies, Marion Arthur Blankenhorn, and Clark Niel Cooper were named by Time magazine as Men of the Year for their contributions.
Nicotinamide adenine dinucleotide (NAD+) was first identified in yeast extracts in 1906 (6), but its redox capabilities were not described until 1936 (7), followed by the connection of reduced NAD (NADH) formation with adenosine triphosphate (ATP) production in 1949 (8). For several decades, research focused on the extensive redox roles of NAD and NAD phosphate (NADP) in animal, plant, and microbial metabolism. An important advance was made in 1966, with the first publication on adenosine diphosphate (ADP)-ribose formation (9). This advance led up to our current knowledge of poly- and mono-ADPribosylation of proteins (10) as well as the formation of cyclic ADP-ribose (11) and of O-acetyl-ADP-ribose by sirtuins (12). These discoveries allowed a much better understanding of the unique metabolic origin of pellagra.
TERMINOLOGY AND CHEMISTRY
The term niacin can have broad or narrow meaning. In the broader sense, as in the “niacin content of a diet,” it could refer to the combination of nicotinic acid and free and nucleotide-bound nicotinamide, all of which would directly contribute to niacin status. In its narrow meaning, niacin refers to nicotinic acid, and the term niacin is used in this manner in the extensive literature on the pharmacologic use of nicotinic acid in the treatment of dyslipidemias and other conditions.
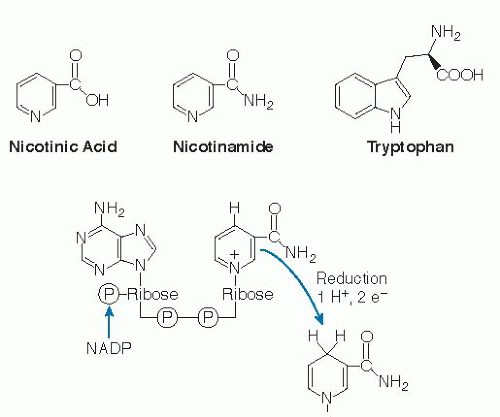
From an ecologic perspective, niacin is introduced into the food chain, predominantly by plants, as nicotinic acid, nicotinamide, and the amino acid tryptophan (Fig. 23.1). Plants often synthesize provitamin metabolites for purposes quite distinct from those of human cells. Plants do use nicotinic acid to form pyridine nucleotides, but plants also use nicotinic acid to form large amounts of alkaloids, such as nicotine (13) and trigonelline (14), for purposes such as pest resistance and growth regulation. Some nicotinamide is formed in plants from nicotinic acid during pyridine nucleotide synthesis and may be released during plant cellular metabolism or during the digestion of plant matter in the human gastrointestinal (GI) tract.
Nicotinic acid and nicotinamide (niacinamide) are position 3 derivatives of the pyridine ring structure (carboxylic acid in the former, carboximide in the latter structure) (see Fig. 23.1). Tryptophan is an essential amino acid in animals, synthesized in plants as a derivative of an indole structure. Despite the differences in ring structure, tryptophan is used to form niacin in many plants (15), and it is used to form NAD+ in the liver of animals, with variable efficiency and poor control with respect to niacin status (16, 17).
The biologically active forms of niacin compounds are the NAD and NADP coenzymes (see Fig. 23.1). The C-4 position on the pyridine ring of the nicotinamide moiety participates in oxidation and reduction reactions. Because of the electronegativity of the amide group and the nitrogen at position 1 on this ring, hydride ions can readily reduce the oxidized C-4 position. This is the basis for the enzymatic hydrogen transfer reactions that are ubiquitous among organisms. With respect to the nonredox functions of NAD, the glycosidic linkage between nicotinamide and ribose is a high-energy bond, and cleavage of this bond drives all types of ADP-ribose transfer reactions in the forward direction.
The oxidized and reduced forms of the coenzymes are designated NAD+ or NADP+ and NADH or NADPH, respectively. The designations NAD and NADP are used to describe the total pools. This is often necessary if the method of quantification does not distinguish between oxidized and reduced forms or if a general statement about the nucleotide pool is made. The total pool of all four forms may be referred to as NAD(P). NAD and NADP have strong ultraviolet absorption at 340 nm in their reduced forms, and this is often used to monitor the oxidation or reduction of these cofactors in enzyme assays.
DIETARY SOURCES
Several categories of foods are good sources of niacin by different mechanisms. Starting with plant-based foods, nuts, legumes, and grains have approximately 2 to 5 mg per average serving, and they are important sources, given the consumption level of these staple foods. Niacin in these foods is largely in the form of nicotinic acid, in some cases bound in poorly available structures as seen in corn. Muscle-based foods, such as poultry, beef, and fish, provide approximately 5 to 10 mg per average serving, mainly in the form of preformed nucleotides, which release nicotinamide during digestion. A third category of niacin-rich foods is created through fortification, usually of flour and cereal products. In Canada and the United States, these products are fortified with approximately 5 mg/100 g flour. The eventual niacin content of ready-to-eat breakfast cereals can range up to 60 mg/100 g of
dry cereal, however, according to the US Department of Agriculture National Nutrient Database (18).
dry cereal, however, according to the US Department of Agriculture National Nutrient Database (18).
The last category of niacin-rich foods consists of highprotein foods that provide tryptophan, converted at low efficiency in the liver to NAD. The contribution of tryptophan is not generally included in the niacin content of a food, but it is included in a calculation of niacin equivalents (1 NE = 1 mg niacin = 60 mg tryptophan, or milligrams niacin + milligrams tryptophan/60). The efficiency of tryptophan conversion is not easily predictable because it will be less efficient with low tryptophan intakes (16, 17).
Niacin in plant products is present mainly in the form of nicotinic acid, although much of it exists in poorly understood bound forms. These bound forms have been studied in wheat bran, corn, and other grains, and they are heterogeneous mixtures of polysaccharides and glycopeptides to which nicotinic acid is esterified (2). In corn, most nicotinic acid is bound, and the tryptophan content is low, thus making pellagra a likely outcome when corn is consumed as a staple grain without alkaline processing. These conditions still occur in developing countries, and outbreaks of pellagra are periodically described (19). Conversely, in the United States, the average daily intake of niacin climbed from approximately 16 mg in the 1930s to approximately 32 mg in 2004 (20) as a result of fortification and increased intake of cereal products. Thus, the incidence of clinically obvious pellagra in developed countries is extremely low. Some evidence still points to subclinical deficiencies in developed countries, however, based on low blood NAD/NADP ratios (21). Niacin deficiency and clinical signs of pellagra may appear in combination with other conditions including anorexia nervosa (22), alcoholism (23), acquired immunodeficiency syndrome (24), cancer (25), and chemotherapy (26).
RECOMMENDED DIETARY ALLOWANCES AND TOLERABLE UPPER INTAKE LIMITS
The Dietary Reference Intake (DRI) values adopted by the United States and Canada include recommended dietary allowances (RDAs), which range from 2 to 8 mg/day in infants and children to 14 mg/day in women and 16 mg/day in men (Table 23.1). The tolerable upper intake levels (ULs) range from 10 to 20 mg/day in children up to 35 mg/day in adults. The UL values only apply to niacin supplements plus niacin fortification, and they are based on the nicotinic acid-induced skin flush response. The skin flush is uncomfortable, but it is not directly related to any real health problems. Very few people have persistent skin flush responses to this level of niacin, and most niacin supplements as well as all B-50, B-75, and B-100 complex formulations greatly exceed the stated UL. Niacin supplements up to 500 mg are freely available for purchase.
Physicians prescribe nicotinic acid up to 3000 mg/day to treat dyslipidemias. This treatment can be effective in decreasing low-density lipoprotein cholesterol and increasing high-density lipoprotein cholesterol (27). The strong skin flush responses decrease over time and can be modulated with cyclooxygenase inhibitors, but patients do struggle with compliance. These higher intakes of niacin also cause some side effects other than skin flush including nausea and, in rare cases, liver injury. The doubling in niacin intake between 1930 and 2005 in the United States preceded the increase in obesity and diabetes in children, and intervention trials showed that very large doses of nicotinamide can impair glucose tolerance (20). The relevance of these results to normal variation in dietary niacin status is uncertain.
The pharmacologic effects of high doses of nicotinic acid and nicotinamide occur by some common and some distinct mechanisms (28), and their deleterious effects need to be researched and assessed separately. Even single effects, such as inhibition of poly(ADP-ribose) polymerases, may have both beneficial and harmful effects on health (29). Very high levels of niacin intake can stress methyl donor status (30) and increase blood homocysteine levels (31). At this point, it is clear that the current UL values are not being enforced, and the potential exists for toxicity of higher doses of nicotinic acid or nicotinamide supplements. Further work should be done to define more valid upper limits, which can then be applied in the marketplace.
SITES OF INTESTINAL ABSORPTION, BLOOD TRANSPORT, AND INTRACELLULAR FORMS
Preformed nicotinamide and nicotinic acid can be absorbed slowly through the stomach lining, but absorption in the small intestine is more rapid. Intact nucleotides are degraded in the upper small intestine to form free nicotinamide. The mechanisms of intestinal absorption
are not fully clear in the current literature. Low concentrations of nicotinic acid and nicotinamide may be transported by sodium-dependent facilitated diffusion (32) or by proton cotransporters (33) or anion antiporters (34). Higher concentrations of both forms appear to be absorbed by passive diffusion, which will come into play with supplement use.
are not fully clear in the current literature. Low concentrations of nicotinic acid and nicotinamide may be transported by sodium-dependent facilitated diffusion (32) or by proton cotransporters (33) or anion antiporters (34). Higher concentrations of both forms appear to be absorbed by passive diffusion, which will come into play with supplement use.
TABLE 23.1 RECOMMENDED DIETARY ALLOWANCES FOR NIACINa | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
Once absorbed from the lumen into the intestinal mucosa, nicotinamide may be converted to NAD (Fig. 23.2, reactions 4 and 5) or released into the portal circulation. Conversely, physiologic levels of nicotinic acid are largely converted through the Preiss-Handler pathway to NAD (32) (see Fig. 23.2, reactions 1, 2, and 3). NAD glycohydrolases release nicotinamide into the portal circulation (see Fig. 23.2, reaction 7). The liver then takes up and converts most of the remaining nicotinic acid in the portal blood to NAD, which it cleaves to release nicotinamide as needed into the systemic circulation. Red blood cells also take up nicotinic acid and nicotinamide, thus forming a circulating reserve pool of pyridine nucleotides (35, 36).
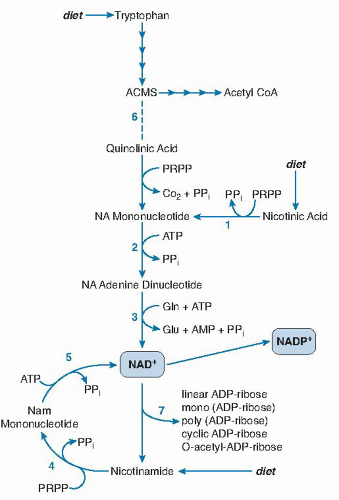 Fig. 23.2. The synthesis and nonredox reactions of pyridine nucleotides. Reactions 1 to 3 constitute the Preiss-Handler pathway for de novo synthesis of nicotinamide adenine dinucleotide (NAD+). Reactions 4 and 5 are used to convert dietary or endogenous nicotinamide into NAD+. Reaction 6 is a spontaneous chemical reaction required for the formation of NAD+ from tryptophan. At position 7 are a large family of varied adenosine diphosphate (ADP)-ribosylation and NAD glycohydrolase reactions. ACMS, 2-amino-3-carboxymuconic-6-semialdehyde; AMP, adenosine monophosphate; ATP, adenosine triphosphate; CoA, coenzyme; Gln, glutamine; NA, nicotinic acid Nam, nicotinamide; PPi, pyrophosphate PRPP, phosphoribosyl pyrophosphate.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|