Overview
The central nervous system (CNS) is the body’s major communication network. Because of localization of functions, neurologic deficits related to disease processes are variable in their presentation. In certain locations of the brain, a lesion will cause minimal or no symptoms, whereas in other areas, it will cause major neurologic deficits. CNS disease can present in many ways, including changes in consciousness, focal neurologic deficits (e.g., aphasia and amnesia, motor and sensory defects), headaches, dizziness, and seizures. Knowledge of the various neurologic pathways will allow a physician to localize the cause of the patient’s symptoms.
CNS diseases are classified within one of many categories, including vascular diseases (e.g., infarcts and spontaneous hemorrhages), trauma, infections, neoplasms, degenerative diseases, toxic and metabolic disorders, and demyelinating diseases. Because the pathology of nervous system diseases is intimately related to their neurologic manifestations, this chapter will begin with a discussion of clinical presentations of central nervous system disorders. This will be followed by a discussion of basic pathologic changes, malformations, vascular diseases, traumatic disorders, infections of the CNS, neoplasms, degenerative diseases, demyelinating disorders, and, finally, a few basic peripheral nerve and skeletal muscle disorders.
Disorders of Consciousness
Requirement for consciousness: Intact and functioning brainstem reticular activating system and its cortical projections.
- Confusion: Impairment of the capacity to think with normal speed and clarity, associated with inattentiveness and disorientation. Delirium is a special example of an acute confusional state in which impaired attention and reasoning are associated with agitation, hallucinations, and in some cases, tremor and convulsions.
- Drowsiness: Inability to remain awake without external stimulation; often associated with some degree of confusion.
- Stupor: State in which only vigorous external stimulation can arouse the patient; once aroused, responses remain markedly impaired.
- Coma: Deep sleep-like state; patient cannot be aroused even with vigorous or repeated external stimulation.
With abnormal CT scan
- Hemispheric mass lesions that cross the midline or impinge upon the brainstem.
- Brainstem lesions that directly affect the reticular formation.
- Subarachnoid hemorrhage.
- Hemispheric mass lesions that cross the midline or impinge upon the brainstem.
With normal CT scan
- Inflammatory disorders, such as bacterial meningitis and viral encephalitis.
- Exogenous toxins, such as sedative drugs, alcohols, opioids, and carbon monoxide.
- Endogenous metabolic insults, such as global hypoxic-ischemic insults, hypoglycemia, hyperammonemia, and hypercalcemia.
- Postictal state.
- Selective brainstem ischemia.
- Inflammatory disorders, such as bacterial meningitis and viral encephalitis.
Diagnosis of cause of changes in consciousness: Establishing a differential diagnosis for the cause of a patient’s change in consciousness requires evaluation of the history preceding the change, the physical examination, and the effectiveness of initial empirical therapy.
- Toxic and metabolic (e.g., opiate overdose, alcohol).
- Infectious (e.g., meningitis, encephalitis, septic shock).
- Cerebrovascular (e.g., stroke).
- Trauma.
- Other (e.g., seizures, neoplasms).
- Preceding headache suggests meningitis, subarachnoid hemorrhage, or encephalitis.
- Preceding intoxication, confusion, or delirium suggests a diffuse process such as meningitis, endogenous metabolic insults, or exogenous toxins.
- Sudden onset of coma suggests brainstem infarct or hemorrhage (e.g., subarachnoid hemorrhage).
- Localizing signs: Suggest focal lesion.
- No localizing signs: Suggests encephalopathy as a result of either an exogenous toxin or an endogenous metabolic insult.
Asymmetrical or reflex functioning of the motor system indicates a focal mass lesion. Changes in pupillary size and reflexes are also useful in assessing the cause of coma.
- A unilaterally dilated nonreactive pupil suggests oculomotor nerve (CN III) compression by an expanding hemispheric mass.
- Pinpoint minimally reactive pupils suggests compromise of the pontine tegmentum (e.g., in a pontine hemorrhage); may also be seen in opiate intoxication. Small but reactive pupils are a feature of many metabolic encephalopathies.
- Minimally reactive pupils in a mid or slightly dilated position suggests a midbrain lesion.
- Bilaterally dilated, nonreactive pupils can be seen in cases of damage to the midbrain tectum or in global ischemic brain injury. It may also be caused by atropine and similar anticholinergic agents.
Localized Cortical Defects
Overview: Focal lesions within the brain can cause symptoms that help the physician localize the lesion to a specific site. These localizing cortical defects include weakness, visual changes, decreased sensation, and a few specialized conditions such as aphasia and amnesia, and others conditions including agnosia and apraxia. Aphasia and amnesia are described in this section.
Basic description: Aphasia is impairment or loss of language function due to damage to language centers in the dominant hemisphere (usually the left). In addition to the two forms of aphasia (Broca and Wernicke aphasia) described below, there are many other forms, such as conduction aphasias caused by lesions to the arcuate fasciculus and global aphasia.
Broca (expressive) aphasia
Basic description: Disorder of fluency of speech affecting both speech and writing. Patients speak with “broken sentences” but comprehension is intact.
Association: Contralateral arm and face weakness due to the proximity of Broca’s area to the motor cortex.
Area involved to produce Broca aphasia: Dominant hemisphere (usually left), inferior frontal lobe.
Wernicke (receptive) aphasia
Basic description: Impairment of comprehension of written and spoken language. Patient can speak but sentences are meaningless (i.e., “wordy sentences”).
Area involved to produce Wernicke aphasia: Inferior superior temporal cortex of the dominant hemisphere.
Overview: In general terms, amnesia indicates the presence of a memory deficit. With anterograde amnesia, patients cannot form new memories. This type of amnesia commonly occurs after cerebral trauma or with dementia. With retrograde amnesia, patients cannot recall past events. Areas of the brain most commonly affected in patients with amnesia include the hippocampus and the diencephalon (hypothalamus, thalamus). One specific form of amnesia discussed here is Korsakoff syndrome.
Korsakoff syndrome
- Cause: Untreated Wernicke encephalopathy (due to thiamine deficiency); most commonly associated with chronic alcoholism.
- Symptoms: Anterograde amnesia, confabulation, ophthalmoplegia, ataxia.
Headache
Dizziness
- Vertigo: Rotational sensation.
- Presyncope: Lightheadedness, near fainting, dimming of vision.
- Disequilibrium: Dysfunctional balance or gait.
- Nystagmus: Involuntary drifting eye movements accompanied by rapid corrective movement. Direction can be horizontal, vertical, rotatory, or mixed. Pure vertical nystagmus is almost always associated with a central lesion.
- Peripheral vertigo: Affecting the inner ear, cochlear apparatus, or vestibulocochlear nerve (CN VIII). Vestibular neuromas are classic but uncommon. In peripheral lesions, the fast phase of nystagmus is away from the affected side.
- Central vertigo: Causes include ischemia, hemorrhage, or infarction; occasionally demyelinating diseases or mass lesions.
Seizures
Basic description of epilepsy: Disease characterized by recurrent paroxysmal electrical discharges in the cerebral cortex known as “seizures.” Manifestations of seizures are variable and include abnormal motor activity, abnormal sensory experiences, and episodic changes in the level of consciousness.
Epidemiology: Seizures can begin at any time during the lifespan; 2–4% of the population has seizures.
Causes of seizures: The incidence of seizures has a bimodal distribution, with seizures in younger patients most often related to epilepsy. The increase in incidence in the elderly population is most often related to cerebrovascular disease and stroke. Most primary seizure disorders are idiopathic and are labeled broadly as epilepsy. Secondary causes of seizures include traumatic brain injury, febrile seizures in children, and tumors.
General Morphologic Changes in the CNS in Response to Injury
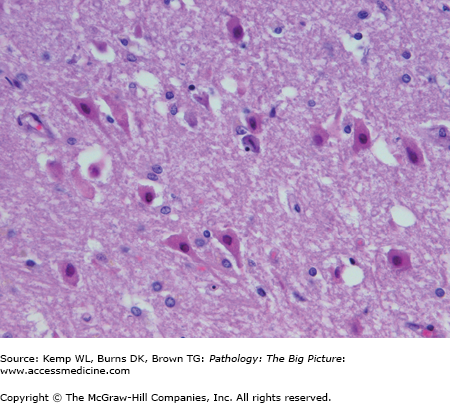
- Acute changes: “Red” neurons (i.e., neurons with hypereosinophilic cytoplasm and pyknotic nuclei (Figure 11-1); usually caused by ischemic injury.
- Subacute and chronic changes: Neuronal loss, inclusions; causes are degenerative diseases.
- Gliosis: Reactive astrocytes with prominent eosinophilic processes.
- Swelling.
- Rosenthal fibers: Brightly eosinophilic elongated structures occurring in response to long-standing gliosis as well as several slow-growing tumors.
Hydrocephalus and Related Disorders
Overview: Hydrocephalus is dilation of the ventricular system and accumulation of excessive cerebrospinal fluid (CSF). It is most often due to impaired resorption of CSF, and is rarely due to overproduction of CSF.
- The two main forms of hydrocephalus are communicating and noncommunicating hydrocephalus, and the distinction between the two is based upon the location of obstruction to CSF flow (Figure 11-2). Communicating and noncommunicating hydrocephalus and hydrocephalus ex vacuo, which are general conditions due to one of several underlying causes, are discussed in this section. The other two forms of hydrocephalus mentioned below are specific conditions.
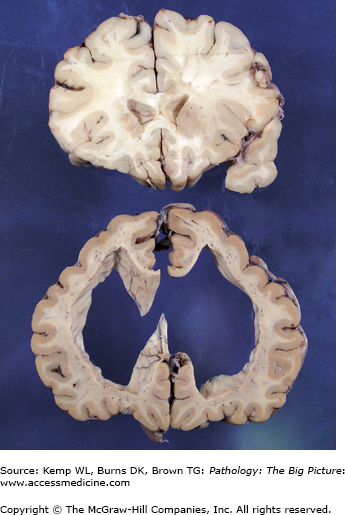
Figure 11-2.
Hydrocephalus (bottom) versus the normal brain (top). Both sections in this image were taken from approximately the same region of two different brains. The section of the brain at the top is normal, and the section at the bottom exhibits marked hydrocephalus. Determining a communicating versus a noncommunicating hydrocephalus based upon one section of brain alone would be difficult unless a process such as fibrosis or hemorrhage were identified in the meninges (causing a communicating hydrocephalus), or the section included the lesion causing obstruction of the ventricular system (causing a noncommunicating hydrocephalus). In addition to obstructive processes, a loss of parenchyma may also cause enlargement of the ventricular system. Dilatation of the ventricular system secondary to tissue loss is usually referred to as hydrocephalus ex vacuo.
- Mechanism: CSF is unable to drain from the subarachnoid granulations into the dural sinuses.
- Causes: Include remote meningitis with scarring; chronic subdural hemorrhage.
- Mechanism: An obstruction exists somewhere within the ventricular system.
- Causes: Include a tumor in the third ventricle or one that obstructs the foramen of Munro; a tumor of the cerebral aqueduct; or, in infants, a malformed or stenotic aqueduct.
- Mechanism: A compensatory dilation of the ventricles in response to a loss of cerebral parenchyma. Not typically associated with increased intracranial pressure.
- Causes: Include Alzheimer disease or a stroke.
Normal pressure hydrocephalus: Patients have dilated cerebral ventricles associated with a normal intracranial pressure. Normal pressure hydrocephalus causes dementia.
Cerebral Edema and Herniation
Overview: Because of the rigid nature of the cranial vault and dural reflections, the brain has only a limited space in which to expand. Expansion of the brain is associated with a variety of mechanical deformations, including flattening of the gyri (Figure 11-3) and several types of herniation patterns. Although cerebral edema can be a generalized process, expansion of the brain can occur as a local process due to a wide variety of causes, including neoplasms and hemorrhage.
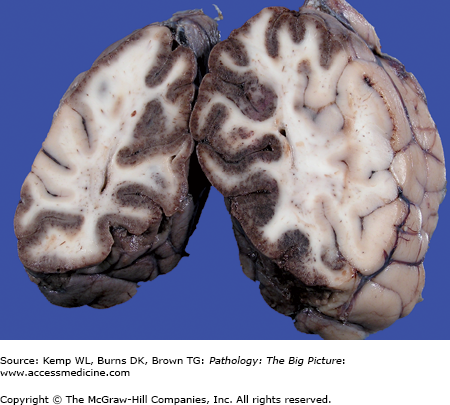
Figure 11-3.
Cerebral edema due to subarachnoid hemorrhage versus the normal brain. The brain on the right side had a subarachnoid hemorrhage, which caused cerebral edema. Note the flattened gyri and narrowed sulci. In comparison, the gyral surfaces of the normal brain on the left are gently rounded, and the intervening sulci are well preserved.
Overview: The two types of cerebral edema are vasogenic and cytotoxic edema, both of which are explained below. However, in many clinical situations, edema is caused by both cytotoxic and vasogenic mechanisms.
Vasogenic edema
- Basic description: Edema occurring as a result of disruption of the integrity of the blood-brain barrier, allowing fluid to escape into the interstitial tissue. The brain has no lymphatics.
- Examples: Neoplasms, abscesses, infarcts.
- Basic description: Edema occurring as a result of disruption of the integrity of the blood-brain barrier, allowing fluid to escape into the interstitial tissue. The brain has no lymphatics.
Cytotoxic edema
- Basic description: Increase in intracellular fluid due to cellular injury.
- Examples: Generalized ischemic injury, infarcts.
- Basic description: Increase in intracellular fluid due to cellular injury.
Complications of cerebral edema
- Cerebellar tonsillar herniation (Figure 11-4): Cerebellar tonsils herniate down the foramen magnum and press on the brainstem. A cerebellar tonsillar herniation is almost always associated with damage to adjacent brainstem structures that control respiration and heart beat, and for that reason, this type of herniation is associated with a high mortality rate.
- Uncal (transtentorial) herniation (see Figure 11-4): The uncus (on the medial aspect of the temporal lobe) herniates under the tentorium cerebelli. The uncus can compress the oculomotor nerve (CN III), compromising the parasympathetic fibers and resulting in ipsilateral pupillary dilation with associated ophthalmoplegia (“down and out”). The uncus can also compress the posterior cerebral artery, causing an ipsilateral occipital lobe infarct (Figure 11-5). Cheyne-Stokes respirations are commonly seen with mesial temporal transtentorial herniation.
- Cingulate (subfalcine) herniation: The cingulate gyrus herniates under the falx cerebri. The anterior cerebral artery may be compressed, causing an infarct of the frontal or parietal lobe in the midline.
- Transcalvarial herniation: When cerebral edema develops in a patient after surgery or trauma, the brain can herniate through the surgical defect in the skull.
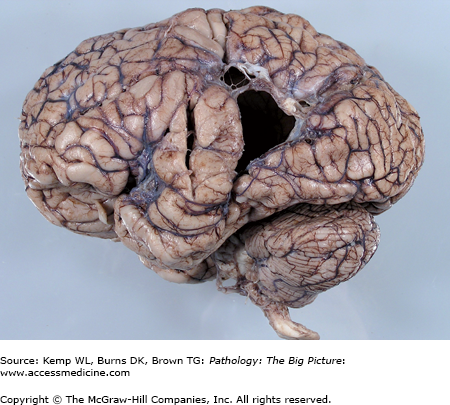
Figure 11-4.
Cerebellar tonsillar and uncal herniation. Increased intracranial pressure in this case has displaced the ventral portion of the cerebellum downward through the foramen magnum, compressing the cerebellar tonsils (arrow). The tonsillar parenchyma is soft and fragmented due to ischemic injury associated with mechanical compression. In addition, the uncal gyri, located on the medial aspect of each temporal lobe, have been pushed against the free leaflet of the tentorium cerebelli, causing prominent grooves to form in these structures (arrowhead).
Figure 11-5.
Bilateral occipital lobe infarcts due to uncal herniation. The red discoloration of the medial gray matter of both occipital lobes is secondary to ischemia induced by compression of the posterior cerebral arteries. Uncal herniation compresses the posterior cerebral arteries, and can also impinge upon the oculomotor nerve (CN III), damaging the parasympathetic nerves and causing ipsilateral pupillary dilation.
Associated condition: Secondary brainstem (Duret) hemorrhage.
- Mechanism of formation of Duret hemorrhage: Kinking of branches of the basilar artery during downward displacement of the brainstem.
- Gross morphology: Midline and paramedian streak-like hemorrhage in the brainstem, oriented dorsal to ventral (Figure 11-6).
Figure 11-6.
Duret hemorrhage. This section of the midbrain contains a secondary brainstem, or Duret hemorrhage, visible here as a midline linear focus of hemorrhage. A secondary brainstem hemorrhage is believed to result from downward displacement of the brainstem, resulting in kinking of branches of the basilar artery and hemorrhagic necrosis of the brainstem parenchyma supplied by these branches.
Malformations
Overview: Most CNS malformations are classified in one of several categories, including neural tube defects, malformations associated with hydrocephalus (Chiari types I and II), disorders of forebrain development, and disorders of neuronal migration. Each of these categories will be discussed below, with examples of each.
Risk factors: Include maternal folate deficiency, incompletely defined geographic and other environmental factors, and gender (incidence is greater in females than in males).
Laboratory testing: Increased level of α-fetoprotein.
Examples of neural tube defects: anencephaly and spina bifida
Anencephaly (Figure 11-7)
Figure 11-7.
Anencephaly. Anencephaly represents one of the most extreme forms of neural tube defect. In this lateral view, one can appreciate the nearly complete absence of the cranial vault and brain above the level of the ears and eyes. As might be expected, such extreme defects are incompatible with prolonged extrauterine survival.
Mechanism of formation: Failure of closure of the anterior neuropore.
Manifestation: Failure of development of the brain and the calvarium.
Incidence: 2–3 in 1000 live births.
Spina bifida
Mechanism of formation: The more severe forms of spina bifida are the result of failure of closure of the posterior neuropore. Less severe forms, such as spina bifida occulta, are the result of failure of secondary neurulation and failure of tail bud formation.
Manifestation: Varies from spina bifida occulta (i.e., incomplete vertebral arch) to myelomeningocele, which has prolapse of the meninges and spinal cord through the defect in the vertebral arch. The most common finding in spina bifida occulta is an abnormal growth of hair over the affected vertebral segment.
Symptoms of spina bifida: Some patients are asymptomatic, whereas other patients have motor and sensory dysfunction and poor bowel and bladder control. The symptoms manifested depend upon the severity of the defect.
Chiari type I malformation
Gross morphology: Herniation of elongated segment of the inferior cerebellar vermis and paravermal folia through the foramen magnum.
Complications: Many patients are asymptomatic; however, the lesion is associated with cranial nerve abnormalities, sudden death, and, in adults, with cerebellar ataxia and late-onset hydrocephalus. Approximately 90% of cases of idiopathic syringomyelia (an abnormal cavity in the spinal cord) have an associated Chiari type I malformation.
Chiari type II malformation
Gross morphology: Abnormally shallow posterior cranial fossa associated with a caudal “herniation” of the medulla and portions of the cerebellar vermis through the foramen magnum.
Complications: Hydrocephalus.
Important point: Almost all cases of Chiari type II malformations are associated with a meningomyelocele in the lumbosacral spinal cord.
Overview: Disorders of forebrain development include holoprosencephaly, agenesis of corpus callosum, and absence of septum pellucidum. Only holoprosencephaly will be discussed here.
Basic description: Malformation resulting from abnormal growth and separation of the developing forebrain.
Incidence: 1 in 30,000 births. Most cases are sporadic, but familial forms also occur.
Risk factors: Certain maternal and environmental factors (e.g., maternal diabetes mellitus, fetal alcohol syndrome, congenital infections), trisomy 13, and mutations in the ZIC2 gene on chromosome 13q. Other cases of holoprosencephaly have been mapped to several additional genetic loci.
Morphology of three types of holoprosencephaly
- Alobar holoprosencephaly: The brain has no hemispheric development. A rudimentary forebrain overlies a common ventricular cavity. The straight gyri, olfactory structures, and commissural structures (e.g., corpus callosum) are absent. There are also severe facial abnormalities (e.g., cyclopia).
- Semilobar holoprosencephaly: Some development of an interhemispheric fissure; straight gyri, olfactory structures, and corpus callosum usually are still absent.
- Lobar holoprosencephaly: Interhemispheric fissure is present, and individual lobes are distinguishable; however, there is some continuity of the cerebral cortex in the midline and aplasia or dysplasia of the corpus callosum.
- Agyria (lissencephaly): The brain is virtually devoid of surface convolutions expected for age.
- Pachygyria: Reduced numbers of gyri, and gyri present are abnormally broad.
- Polymicrogyria: Numerous abnormally convoluted gyri.
- Other disorders of neuronal migration: Neuronal heterotopias, white matter heterotopias.
Acquired CNS Abnormalities in Neonates
Overview: Primary malformations may be difficult to distinguish from acquired injury, because prior to 16 weeks’ gestational age, the brain does not mount the usual reaction to injuries. Germinal matrix hemorrhage, periventricular leukomalacia, porencephaly, and multicystic encephalopathy are discussed in this section.
- Morphology: Chalky discoloration of the white matter associated with hemorrhage and cavitation.
- Risk factors: Hyaline membrane disease, shock, sepsis, and congenital heart disease.
- Important point: PVL is often seen in association with cerebral palsy.
Porencephaly: Cystic defect with a smooth contour that allows the ventricular system to communicate with the subarachnoid space (Figure 11-8). Most examples likely represent sequelae of intrauterine ischemic injury early in gestation.
Vascular Malformations
- Morphology: Mass of enlarged arteries, veins, and hybrid channels, with intervening nervous tissue.
- Epidemiology: 10–30 years of age; male predominance.
- Location: Most occur along the distribution of the middle cerebral artery.
- Complications of arteriovenous malformation: Seizure disorder, intracerebral hemorrhage, subarachnoid hemorrhage.
Cavernous angioma: Dilated venous spaces (no arterial component) with no intervening nervous tissue; these malformations may bleed.
Vascular Diseases
Overview: Cerebrovascular disease is the third main cause of death in adults. The most common causes are thrombosis, emboli, and hemorrhage. Cerebrovascular disease can be generalized, as might be seen following cardiac arrest (global hypoxic ischemic encephalopathy), or it can be localized (cerebral infarct). The term “stroke” is used to designate the sudden onset of a localized, nonconvulsive, neurologic deficit. Strokes can be caused by either a cerebral infarct or a primary (nontraumatic) hemorrhage. Stroke, global hypoxic-ischemic encephalopathy, cerebral infarcts, and intracerebral hemorrhage will be discussed in this section.
Basic description of stroke: Term used when CNS symptoms begin suddenly and persist for more than 24 hours. As noted, strokes can be caused by ischemic injury or by primary hemorrhage; 80% are ischemic in origin.
Basic description of transient ischemic attack (TIA): Brief episode of neurologic dysfunction relating to a specific vascular bed that lasts less than 24 hours. TIAs are usually caused by small emboli. The risk of having a future stroke following a TIA is 15% within 1 year and 25% within 2 years.
Clinical presentation of stroke: Acute spontaneous onset of central, localizing, neurologic dysfunction of more than 24 hours’ duration.
Basic description: Condition resulting from generalized poor perfusion of the brain, and hence is probably more properly termed “ischemic” encephalopathy. Some areas of the brain are more sensitive than others.
Cause: Global cerebral ischemia.
Areas most commonly affected by global hypoxic-ischemic encephalopathy in adults
- Arterial borderzone areas (i.e., regions between the distribution of the cerebral arteries).
- Hippocampus (particularly the so-called CA1 region of the pyramidal cell layer).
- Purkinje cells and dentate nucleus in cerebellum.
- The basal ganglia and thalamus are other common sites of injury.
Morphology of global hypoxic-ischemic encephalopathy
- Gross: Appearance depends upon time period since injury; can have edema, dusky red discoloration (particularly if there has been reperfusion), and laminar cortical necrosis (midcortical layer neurons are most susceptible to ischemic injury). In the cerebral cortex, ischemic injury is almost always more pronounced at the depths of sulci than in the gyral crests.
- Microscopic: At 12–24 hours, “red” neurons (i.e., neurons with eosinophilic cytoplasm and pyknotic nuclei). There is usually minimal host inflammatory reaction due to lack of perfusion of the brain.
Basic description: Focal irreversible brain injury resulting from a localized loss of blood flow.
Causes of cerebral infarcts
- In the anterior circulation, most commonly middle cerebral artery territory, infarcts are usually due to emboli from atherosclerotic plaques in the carotid arteries.
- In the posterior circulation, infarcts are usually from thrombi due to atherosclerosis. Vasculitis (either infectious or noninfectious) and trauma are less common causes.
Types of cerebral infarcts
- Hemorrhagic infarct: Causes are usually emboli or conditions associated with compression of the vessels such as herniations.
- Nonhemorrhagic infarct: Cause is usually thrombi.
Three stages of morphologic development of a cerebral infarct (Table 11-1)
- Acute.
- Organizing (changes develop beginning about day 3).
- Remote (changes develop within 6 months after infarct).
Stage of Infarct | Gross | Microscopic |
|---|---|---|
Acute | Poorly defined area of soft parenchyma; preservation of architecture | Red neurons Rarefaction of white matter |
Organizing | Well-defined area of soft, friable parenchyma; loss of architecture | Sheets of foamy macrophages |
Remote | Cystic lesion | Gliosis at periphery |
Morphology of acute infarcts
- Gross: Soft parenchyma with preservation of architecture. Hemorrhagic infarcts have petechial hemorrhages and larger hemorrhages as a result of reperfusion (Figure 11-9).
- Microscopic: Red neurons and rarefaction of white matter (within 12–24 hours) are associated with a neutrophilic infiltrate. In hemorrhagic infarcts, there are extravasated red blood cells as a result of reperfusion.
Figure 11-9.
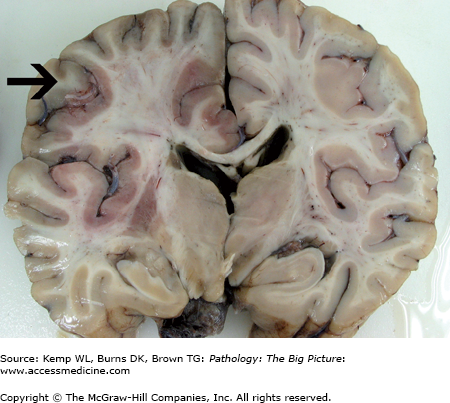
Acute cerebral infarct. Much of the left cerebral hemisphere is expanded by an area of acute necrosis in the distribution of the left middle and anterior cerebral arteries. Note the subtle dusky discoloration of much of the medial, superior, and lateral cerebral cortex on the left (black arrow) caused by leakage of small amounts of blood into the necrotic parenchyma (some of the red discoloration in the deeper areas of the brain is caused by incomplete fixation of the necrotic tissue). The infarct was caused by a surgical procedure that compromised the left internal carotid artery. Microscopic examination of the infarct at this stage would reveal red neurons. Also note herniation of the left cingulate gyrus and left uncus, which was caused by edema associated with the infarct.
Morphology of organizing infarcts
- Gross: Soft, friable parenchyma with loss of architecture; the lesion becomes more sharply defined over time (Figure 11-10).
- Microscopic: Foamy macrophages containing engulfed myelin debris begin to infiltrate within 48–72 hours and remain in the area for about 6 months (Figures 11-11, 11-12, 11-13).
Figure 11-10.
Organizing cerebral infarct. In the area of the right insula is a well-demarcated cystic lesion involving the insular cortex, fronto-parietal white matter, and portions of the basal ganglia. This process is an organizing cerebral infarct. The lesion is well demarcated because the sheets of macrophages, which are engulfing dead neurons and broken-down myelin, end abruptly at the viable tissue. Once the dead tissue is completely removed, only a cavity will remain.
Figure 11-11.
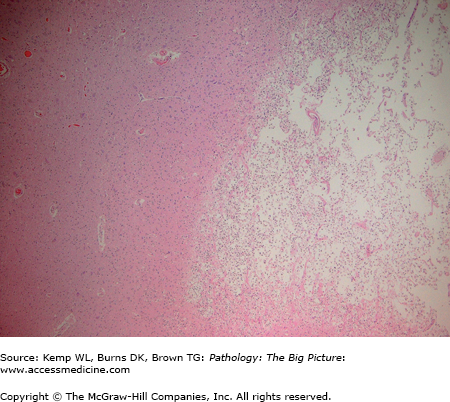
Organizing cerebral infarct. This lower power photomicrograph illustrates an organizing cerebral infarct. The right side of the image has sheets of lipid-laden macrophages, and the left side of the image has viable white matter. Note the clear line of demarcation between the two components. Hematoxylin and eosin, 40×.
Figure 11-12.
Organizing cerebral infarct. This higher power photomicrograph of an organizing cerebral infarct better shows the cellular morphology, with lipid-laden macrophages to the left side of the image and viable neurons on the right side of the image. In the white matter, at the junction, are a few astrocytes with prominent eosinophilic stellate cytoplasmic processes (arrow). The term “gliosis” is often used to designate an accumulation of reactive astrocytes of this type. Hematoxylin and eosin, 200×.
Figure 11-13.
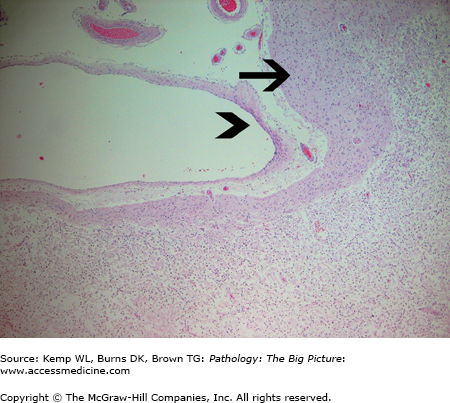
Organizing cerebral infarct with subpial sparing. The subpial gray matter (arrow) receives its nutrients from meningeal vessels and cerebrospinal fluid, and thus survives ischemic injury induced by thrombosis or an embolus involving the cerebral arteries. The arrowhead indicates the pia mater. Underlying the subpial white matter is an organizing cerebral infarct containing sheets of lipid-laden macrophages. This pattern of subpial sparing is sometimes helpful in distinguishing infarcts from contusions of the brain. Hematoxylin and eosin, 40×.
Morphology of remote infarcts
- Gross: Cystic lesion (Figure 11-14).
- Microscopic: Gliosis. If the infarct is near the cortical surface, there is subpial sparing because the subpial parenchyma receives its oxygen and nutrients from the CSF and meningeal vessels.
Figure 11-14.
Remote cerebral infarct. In the distribution of the left anterior cerebral artery is a remote cerebral infarct involving the medial aspect of the cerebral hemisphere. Remote cerebral infarcts are cavitary, as gliosis does not produce a macroscopically appreciable scar. In the photograph, the cavity has collapsed, imparting an irregular contracted appearance to the surface of the hemisphere.
Clinical presentation of infarcts: Symptoms depend upon the size and location of the infarct.
- Middle cerebral artery distribution: Contralateral hemiparesis and hemisensory loss; the face and upper extremity are more affected than the lower extremity; aphasia if the infarct involves the dominant cerebral hemisphere.
- Anterior cerebral artery distribution: Contralateral hemiparesis and hemisensory loss; the lower extremity is more affected than the upper extremity.
- Posterior cerebral artery distribution: Contralateral hemiparesis, homonymous hemianopsia, amnesia, sensory loss.
- Basilar artery distribution: Quadriparesis, dysarthria, dysphagia, diplopia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree