INTRODUCTION
QUICK REVIEW Organization of Skeletal Muscle
Neuromuscular diseases include a wide-ranging variety of pathologies that can produce significant disability for the patient. The history and physical examination are very important in narrowing the differential, and the muscle biopsy provides an additional layer of granularity to assist in making the diagnosis. To understand muscle pathology, one must understand the organization of the skeletal muscle and the histochemical stains that provide the means necessary to distinguish between potential differentials (Figures 19-1 and 19-2).
Fascicles
Perimysium: Connective tissue surrounding the fascicles
Endomysium: Connective tissue surrounding the muscle fibers inside the fascicles
Epimysium: Connective tissue covers the outer surface of the muscle
Muscle Fibers
Myofibrils: An elongated structure containing cytoskeletal elements allowing the muscle to contract
Sarcomere: Thick (myosin) and thin filaments (actin, troponin, tropomyosin)
The striated muscle appearance is created by pattern of alternating dark and light bands
Sarcolemma (Plasma Membrane)
Sarcoplasma: Specialized cytoplasm of a muscle cell
Nuclei and mitochondria are located just beneath the sarcolemma
Sarcoplasmic reticulum extends between the myofibrils
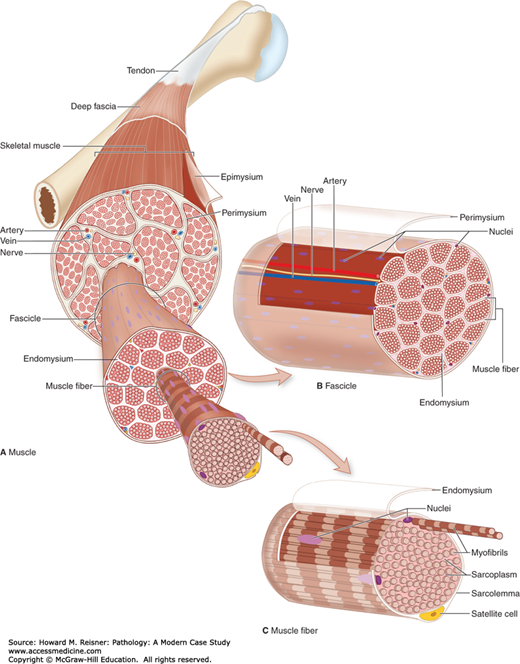
FIGURE 19-1
Organization of skeletal muscle. (A) An entire skeletal muscle is enclosed within a dense connective tissue layer called the epimysium continuous with the tendon binding it to bone. (B) Each fascicle of muscle fibers is wrapped in another connective tissue layer called the perimysium. (C) Individual muscle fibers (elongated multinuclear cells) are surrounded by a very delicate layer called the endomysium, which includes an external lamina produced by the muscle fiber (and enclosing the satellite cells) and ECM produced by fibroblasts. (From Mescher AI, Junqueira’s Basic Histology, 12th ed. McGraw Hill Lange New York 2010, Chapter 10, Figure 10-3, page 170.)
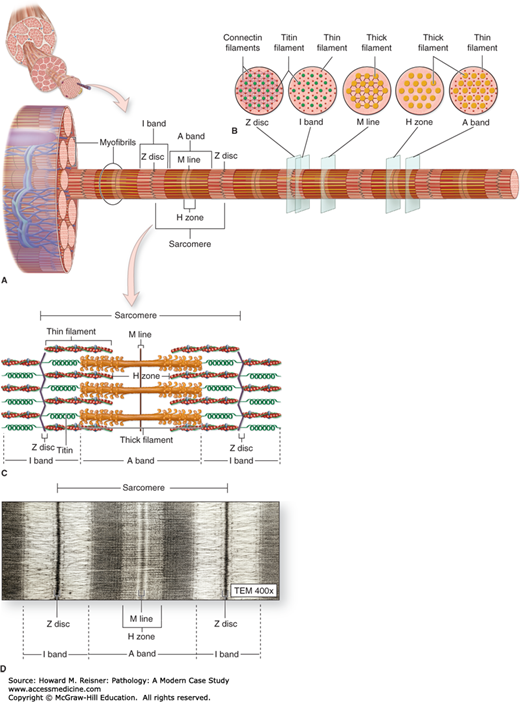
FIGURE 19-2
Structure of a myofibril: a series of sarcomeres. (A) Diagram indicates that each muscle fiber contains several parallel bundles called myofibrils. (B) Each myofibril consists of a long series of sarcomeres that contain thick and thin filaments and are separated from one another by Z discs. (C) Thin filaments are actin filaments with one end bound to α-actinin, the major protein of the Z disc. Thick filaments are bundles of myosin, which span the entire A band and are bound to proteins of the M line and to the Z disc across the I bands by a very large protein called titin, which has spring-like domains. (D) The molecular organization of the sarcomeres has bands of greater and lesser protein density, resulting in staining differences that produce the dark and light-staining bands seen by light microscopy and TEM. (From Mescher AI, Junqueira’s Basic Histology, 12th ed. McGraw Hill Lange New York 2010, Chapter 10 Figure 10-8, page 173.)
WHAT TO DO Evaluation of Myopathies
To appropriately work up a myopathy, the following elements should be included:
Detailed history, including related family medical conditions
Detailed neurological examination especially of the different muscle groups to determine the distribution of weakness
Laboratory testing: CK, liver function tests, ESR, CRP, ANA (antinuclear antigen), anti-double stranded DNA antibody, ENA (extractable nuclear antigens), rheumatoid factor, complement (C3, C4), myositis profile
Genetic testing
Electrophysiological studies
Muscle biopsy
A muscle biopsy includes several investigational components including the following features:
Routine histochemical analysis
Immunostaining
Biochemical analysis
Genetic analysis
Muscle fibers can be divided based on specific characteristics and can be found in different concentrations in different muscles throughout the body. Muscles form a mosaic or checkerboard pattern of different fiber types.
Type 1: Slow-twitch, oxidative
Type 2A: Fast-twitch, oxidative-glycolytic
Type 2B: Fast-twitch, glycolytic
Type 2C: Undifferentiated
Type 1 fibers have the following properties:
Loaded with mitochondria
Depend on cellular respiration for ATP
Fatty acids are the main energy source
Resistant to fatigue
Rich in myoglobin (red meat)
Activated by small diameter, slow conducting motor neurons
Slow twitch fibers
Muscles used in activities requiring endurance
Type 2 fibers have the following properties:
Few mitochondria
Rich in glycogen
Depend on creatine phosphate and glycolysis for ATP production
Low in myoglobin (white meat)
Activated by large diameter fast conducting motor neurons
Fast twitch fibers
Rapid and forceful movement
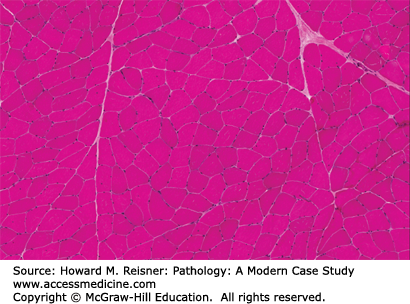
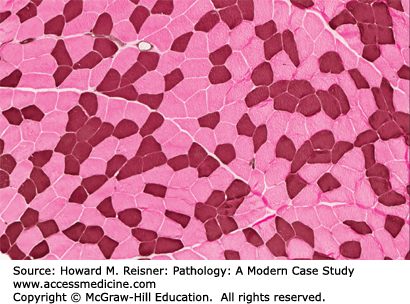
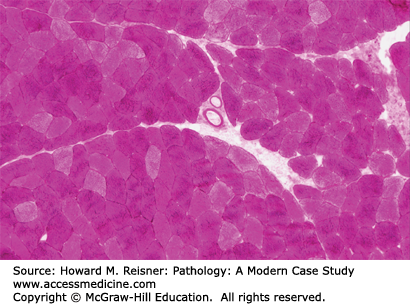
Hematoxylin & eosin (H&E): Enables visualization of the general structure including fiber size, position of nuclei, fibrosis, inflammation, evidence for necrosis, and invasion by macrophages (myophagocytosis) (Figures 19-3, 19-4, 19-5)
Gomori trichrome: Stains mitochondria, nemaline rods, and membranous whorls of rimmed vacuoles red; type 1 fibers are darker
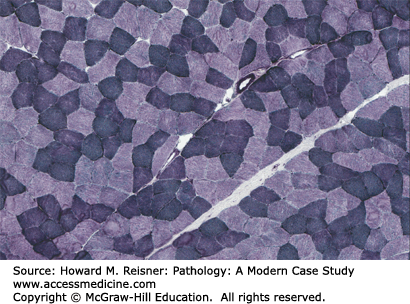
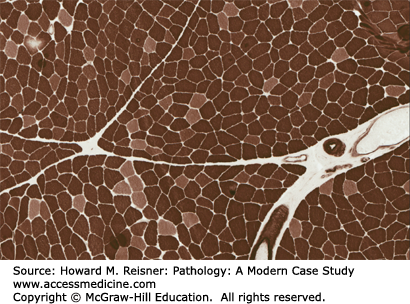
Nicotinamide adenine dinucleotide (NADH): Enables separation of fiber types based on density of mitochondria, reveals distribution of mitochondria, and can show myofibrillar disruption; type 1 fibers are darker (Figures 19-6, 19-7, 19-8).
Adenosine triphosphatase (ATPase) 4.3: Reveals the distribution and involvement of the different fiber types and their subtypes. At this pH type 1 fibers stain darker
Adenosine triphosphatase (ATPase) 9.6: Same as 4.3. At this pH type 2 fibers stain darker
Periodic acid-Schiff (PAS): Heavily stains those fibers with excessive glycogen; fibers with loss of glycogen are white. The example shown is McArdle disease.
Myophosphorylase: Absence of this enzyme in muscle fibers occurs in McArdle disease (myophosphorylase deficiency)
Congo red: Reveals the presence of β-amyloid as seen in inclusion body myositis as discussed below
MUSCLE PATHOLOGY
Neuromuscular diseases demonstrate a wide variety of pathology with regard to muscle biopsies. Each muscle sample provides different clues based on the different stains mentioned above that guide the investigator and help narrow the differential. These clues for the investigator include
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree