Objectives
- Understand the integrated response to a meal and the need for mechanisms that regulate the function of the gastrointestinal tract as a whole
- Describe modes of communication in the gastrointestinal tract
- General features of neurohumoral regulation
- Characteristics of chemical signals
- General features of neurohumoral regulation
- Understand principles of endocrine regulation
- Definition of a hormone
- Identify established and candidate GI hormones and their mechanisms of action
- Definition of a hormone
- Understand the design of the enteric nervous system and neurocrine regulation
- Describe immune and paracrine regulatory pathways
- Understand how the GI tract and the brain cooperate to regulate food intake
Requirement for Integrated Regulation
 As we have learned from the previous chapter, the gastrointestinal system subserves several functions that are critical for whole-body homeostasis. For nutrient assimilation in particular, specific tissues and regions of the gastrointestinal system must sense, signal, and respond to the ingestion of a meal (Figure 2–1). By cooperating with the central nervous system, the gastrointestinal system is also intimately involved in the control of food intake. To conduct the business of the gastrointestinal system most efficiently, the various segments must communicate. Thus, the activities of the gastrointestinal tract and the organs that drain into it are coordinated temporally via the action of a series of chemical mediators, with the system being referred to collectively as neurohumoral regulation, implying the combined action of soluble and neuronal pathways. The integrated regulation of gastrointestinal function underlies the efficiency of the system as described in Chapter 1, and its ability to provide for the effective uptake of nutrients even when they are in short supply.
As we have learned from the previous chapter, the gastrointestinal system subserves several functions that are critical for whole-body homeostasis. For nutrient assimilation in particular, specific tissues and regions of the gastrointestinal system must sense, signal, and respond to the ingestion of a meal (Figure 2–1). By cooperating with the central nervous system, the gastrointestinal system is also intimately involved in the control of food intake. To conduct the business of the gastrointestinal system most efficiently, the various segments must communicate. Thus, the activities of the gastrointestinal tract and the organs that drain into it are coordinated temporally via the action of a series of chemical mediators, with the system being referred to collectively as neurohumoral regulation, implying the combined action of soluble and neuronal pathways. The integrated regulation of gastrointestinal function underlies the efficiency of the system as described in Chapter 1, and its ability to provide for the effective uptake of nutrients even when they are in short supply.
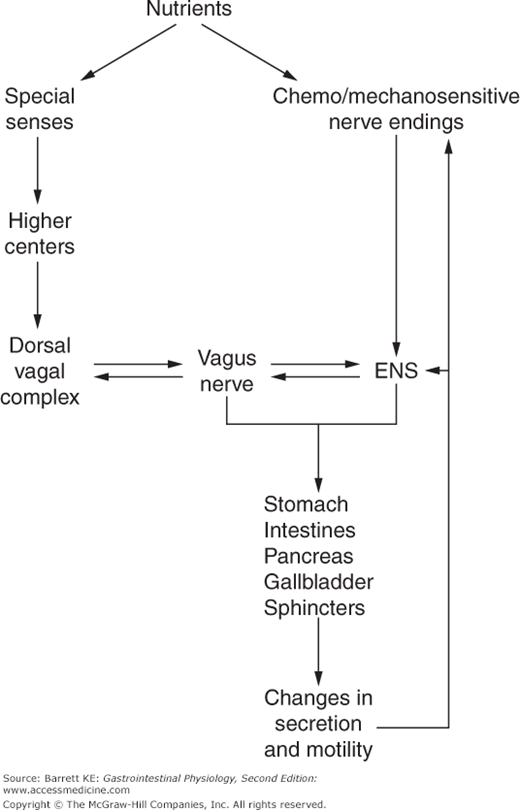
Figure 2–1.
Overview of neural control of the gastrointestinal system. Nutrients activate both special senses (smell, taste) as well as specific sensory nerve endings that exist within the wall of the gut. These responses are conveyed via the autonomic nervous system and enteric nervous system (ENS) to alter the function of the gastrointestinal tract and organs draining into it, resulting in changes in secretion and motility. Such functional changes may additionally feedback on neural control to allow for appropriate homeostasis of the system.
Communication in the GI Tract
The gastrointestinal tract stretches from mouth to anus, implying that communication that rests simply on diffusion of locally released signals will not be adequate for the timely transfer of information from one segment to another. Likewise, the gastrointestinal tract also needs to communicate its status to organs that drain into it, such as the pancreas and gallbladder, and to distant organs, such as the brain. Thus, the system has evolved mechanisms for communication over significant distances, although local messengers also play a role in fine-tuning information delivery or, in some cases, amplifying or antagonizing it. Overall, information is carried between the various sites by chemical entities possessing specific physicochemical properties. Another general principle underlying communication in the gastrointestinal system is that of functional redundancy. Several different mediators may often produce the same physiologic response, and single mediators may alter the function of more than one system.
Neurohumoral regulation is effected by several classes of chemical messengers—peptides, derivatives of amino acids such as histamine and nitric oxide, small molecule neurotransmitters, and lipid mediators such as prostaglandins and steroids. The gastrointestinal tract is a rich source of unique peptides that are synthesized by enteroendocrine cells as well as packaged in nerve endings. In fact, all five of the known gastrointestinal hormones are peptides, but it does not always follow that any gastrointestinal peptide is a hormone. Major gastrointestinal messengers that have definitely been assigned physiologic roles are listed in Table 2–1.
| Endocrine | Neurocrine | Paracrine | Immune/Juxtacrine |
|---|---|---|---|
| Gastrin | Acetylcholine | Histamine | Histamine |
| Cholecystokinin | Vasoactive intestinal polypeptide | Prostaglandins | Cytokines |
| Motilin | Substance P | Somatostatin | Reactive oxygen species |
| Secretin | Nitric oxide | 5-hydroxytryptamine | Adenosine |
| Glucose-dependent insulinotropic peptide | Cholecystokinin | ||
| Ghrelin | 5-hydroxytryptamine | ||
| Somatostatin | |||
| Calcitonin-gene related peptide |
Overproduction of gastrointestinal messengers and their release in an uncontrolled fashion can lead to disease states. For example, Zollinger–Ellison syndrome is the result of a secreting gastrinoma, and results in pathologic increases in gastric acid secretion among other symptoms. Likewise, carcinoid tumors overproduce the paracrine/neurotransmitter 5-hydroxytryptamine (5-HT), also known as serotonin.
Finally, the kinetics of information transfer by any given molecule will also depend on its stability and/or the rate of its reuptake. Several of the gastrointestinal messengers that are designed to act over long distances incorporate structural features that retard their metabolism. Conversely, mediators that act only in the immediate vicinity of their site of release are rapidly degraded and/or actively taken back into nerves for repackaging into secretory vesicles.
Hydrophobic messengers, such as steroids and nitric oxide, can readily traverse cell membranes and thus can interact with intracellular targets. Hydrophilic messengers, such as peptides, many small molecule neurotransmitters, and prostaglandins, on the other hand, use classical receptor/second messenger pathways to mediate their effects.
The majority of the hydrophilic messengers that are relevant to gastrointestinal physiology bind to receptors linked to G-proteins, with consequent increases in intracellular calcium or cyclic AMP (cAMP). The use of different second messengers also provides for potentiation, or synergism, when a given cell is acted on by more than one mediator simultaneously. An example of such synergy is found in the control of acid secretion by gastric parietal cells, as will be covered in detail in Chapter 3. And at least one chemical mediator in the gastrointestinal tract, somatostatin, acts at receptors linked to inhibitory G proteins. These can antagonize increases in cAMP produced by other mediators.
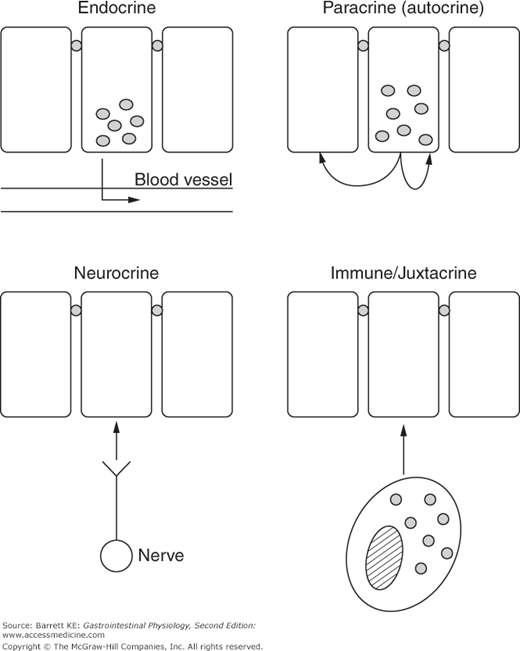
 Four modes of communication are recognized within the gastrointestinal system—endocrine, neurocrine, paracrine (of which autocrine is a special case), and juxtacrine regulation, most often ascribed to cells of the immune system. A diagrammatic representation of each of these is provided in Figure 2–2. Note that these modes subserve different communication needs. Thus endocrine regulation can be considered as “broadcast” regulation that impacts the function of several systems simultaneously. The specificity of this mode of communication is determined by the distribution of receptors for the endocrine messenger, or, to carry forward the broadcast analogy, to those who have their radio receiver tuned in to the specific station carrying the data.
Four modes of communication are recognized within the gastrointestinal system—endocrine, neurocrine, paracrine (of which autocrine is a special case), and juxtacrine regulation, most often ascribed to cells of the immune system. A diagrammatic representation of each of these is provided in Figure 2–2. Note that these modes subserve different communication needs. Thus endocrine regulation can be considered as “broadcast” regulation that impacts the function of several systems simultaneously. The specificity of this mode of communication is determined by the distribution of receptors for the endocrine messenger, or, to carry forward the broadcast analogy, to those who have their radio receiver tuned in to the specific station carrying the data.
Neurocrine communication, on the other hand, can also transmit information over long distances, but is analogous to communication by telephone rather than radio; the specificity is determined by spatial delimitation of the site(s) at which the message is ultimately delivered, based on synapses at target cells. Of course, the target cell is also required to bear an appropriate receptor for the neurotransmitter that is delivered, but in general, nerves do not innervate cells that are unable to respond to the former’s messengers.
Finally, paracrine and immune regulation are usually only effective in the immediate vicinity of mediator release. Thus, these can be considered as modes of communication that are analogous to live conversations between a few individuals.
Because of its ability to regulate multiple sites in an essentially simultaneous fashion, endocrine regulation is critical to the integrated function of the gastrointestinal tract and organs that drain into it in response to a meal. The intestine is extremely well supplied with cell types responsible for the synthesis and release of endocrine mediators, known as hormones; in fact, if all of the endocrine cells within the gut were assembled as a single structure, they would make up the largest endocrine organ in the body. The gastrointestinal hormones were also the first to be discovered, with the identification of secretin by Bayliss and Starling in 1902. We define gastrointestinal hormone here as an endocrine messenger that both is released by and acts upon the gastrointestinal system. However, the intestine also produces other hormones with targets outside the GI system. An example is ghrelin, a stomach peptide that affects feeding behavior via the central nervous system.
Endocrine hormones are packaged within the secretory granules of distinct cell types within the wall of the intestinal tract, and released in response to nervous activity as well as chemical and mechanical signals coincident with food ingestion. The endocrine cells of the gut have been identified with letters to denote their hormonal contents; gastrin, secretin, cholecystokinin, glucose-dependent insulinotropic peptide (also referred to as gastric inhibitory peptide, or GIP), motilin and ghrelin are stored in G, S, I, K, M (or Mo) and A/X cells, respectively. Some endocrine cells may have processes that contact the luminal contents and are activated to release their mediators in response to specific features of luminal composition, such as acidity, osmolarity, or nutrients such as amino acids and free fatty acids. Direct neural control of hormone release by neurotransmitters is also important in some cases. In other cases, hormone release in response to changes in luminal composition can also be activated by a reflex arc that first involves activation of a sensory enteric nerve ending, with subsequent release of specific neurotransmitters close to the surface of the endocrine cell to stimulate exocytosis. Yet other endocrine cells are designed to respond to conditions existing in the interstitium.
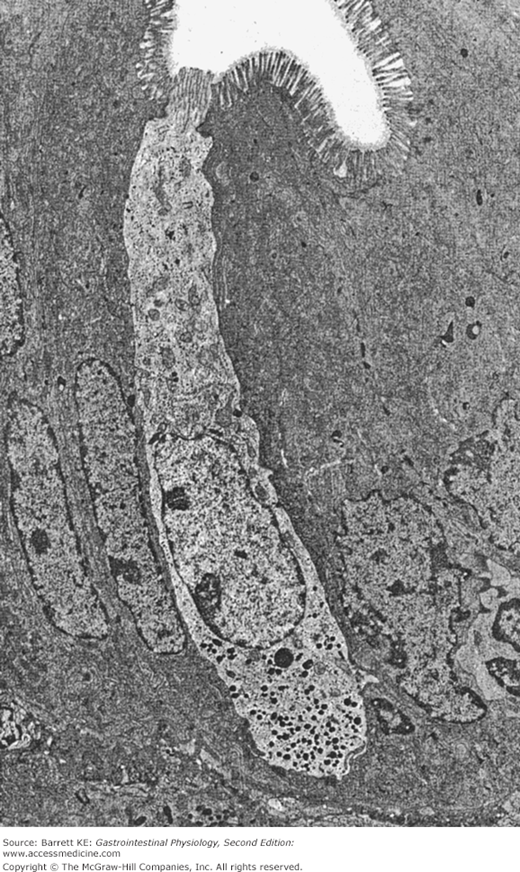
Endocrine cells that contact the lumen are referred to as having an “open” morphology; those that do not are called closed. An electron micrograph showing a typical open enteroendocrine cell is shown in Figure 2–3. Note how the apical pole of the cell is in contact with the lumen, and how the secretory granules are toward the base of the cell, strategically located to release their contents into the lamina propria and thence into the bloodstream.
Figure 2–3.
Electron micrograph of an “open” gastrointestinal endocrine cell in the human jejunum amid several enterocytes. Secretory granules are localized to the basolateral pole of the endocrine cell. (Reproduced with permission from Solcia et al. Endocrine cells of the digestive system. In: Johnson LR, ed. Physiology of the Gastrointestinal Tract. 2nd ed. New York: Raven Press; 1987.)
The hormones that are released from these endocrine cells diffuse into the lamina propria and thence into the portal circulation. From there, they travel to target organs and modify secretion, motility, and cell growth. Most hormones signal to segments of the gastrointestinal tract that are distal to their site of release, but feedback signaling can also occur. For example, cholecystokinin, released from the duodenal mucosa, can signal back to the stomach to retard its emptying. Some hormones additionally act by binding to receptors on nerves such as, for example, on vagal afferents. In this way, their action can be amplified by the simultaneous recruitment of neurocrine regulation as discussed below.
 All of the currently known GI hormones are peptides, but not all peptides isolated from the gastrointestinal tract are hormones. In fact, the GI tract is a very rich source of biologically active peptides, comparable to the central nervous system, but thus far, only five have fulfilled all of the criteria to be considered a hormone, despite intense scrutiny. The criteria that must be fulfilled to define a hormone are listed in Table 2–2. Of these, the structural criterion seems relatively trivial in the days of automated peptide sequencers and synthesizers, but represented a technical tour de force in the early twentieth century when most of the GI hormones were discovered. Other gastrointestinal peptides that have not yet fulfilled all of the criteria listed, yet which are suspected to have physiologic functions following their release, are considered to be “candidate hormones” and several have attracted the interest of the pharmaceutical industry on the basis of their specific properties, as will be discussed below.
All of the currently known GI hormones are peptides, but not all peptides isolated from the gastrointestinal tract are hormones. In fact, the GI tract is a very rich source of biologically active peptides, comparable to the central nervous system, but thus far, only five have fulfilled all of the criteria to be considered a hormone, despite intense scrutiny. The criteria that must be fulfilled to define a hormone are listed in Table 2–2. Of these, the structural criterion seems relatively trivial in the days of automated peptide sequencers and synthesizers, but represented a technical tour de force in the early twentieth century when most of the GI hormones were discovered. Other gastrointestinal peptides that have not yet fulfilled all of the criteria listed, yet which are suspected to have physiologic functions following their release, are considered to be “candidate hormones” and several have attracted the interest of the pharmaceutical industry on the basis of their specific properties, as will be discussed below.
|
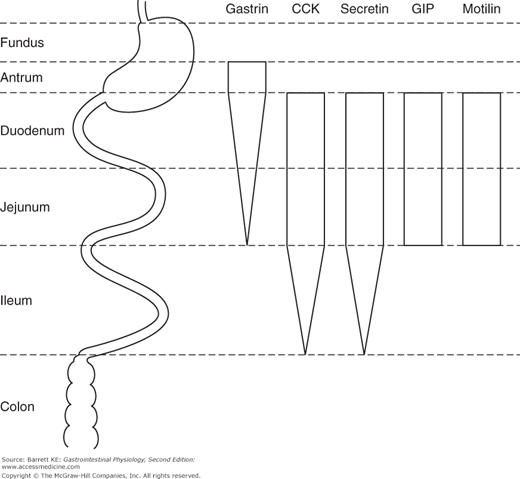
The GI hormones are synthesized in various segments of the gastrointestinal tract (Figure 2–4), but only gastrin appears to be present in the stomach of healthy individuals. The stomach is also the major source of ghrelin, contributing about two-thirds of the body’s production of this hormone. The remaining hormones are present in greatest amounts in the duodenum and jejunum, with tapering expression of cholecystokinin and secretin into the ileum in addition. However, under normal conditions, most of the release of gastrin occurs in the stomach, and of the other hormones in the duodenum and, to some extent, the jejunum. Ileal expression of some hormones, therefore, represents another example of the “reserve capacity” of the intestine that can be called upon to regulate gastrointestinal function if required. Further, in health, there appears to be little if any expression of gastrointestinal hormones in the colon. However, because the endocrine cells that secrete these peptides arise from multipotential stem cells in the gut epithelium, when colonic epithelial tumors also arise, sometimes one or more gastrointestinal hormones are aberrantly expressed. This may have clinical significance in that several of the GI hormones are known to have trophic or growth-promoting effects, and thus may contribute to the unregulated growth of some colon cancers in an autocrine fashion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree