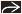Neuroblastoma and Ganglioneuroblastoma
Jessica Comstock, MD
Cyril Fisher, MD, DSc, FRCPath
Key Facts
Terminology
Malignant tumor derived from primordial neural crest cells
Clinical Issues
3rd most common malignant tumor in children
90% diagnosed by age 5 years
Follows distribution of sympathetic ganglia, also adrenal medulla
Presentation depends on age of patient, location of tumor, and associated clinical syndromes
Urine catecholamines elevated in 95% of patients with neuroblastoma (NB)
Microscopic Pathology
International Neuroblastoma Pathology Committee
Classification
Undifferentiated NB
Poorly differentiated NB
Differentiating NB
Nodular GNB
Intermixed GNB
Mitotic-karyorrhectic index (MKI)
Ancillary Tests
N-myc amplification is associated with worse prognosis
Top Differential Diagnoses
Alveolar rhabdomyosarcoma (ARMS)
Ewing sarcoma/primitive neuroectodermal tumor (PNET)
Ganglioneuroma
Lymphoma
TERMINOLOGY
Abbreviations
Neuroblastoma (NB)
Ganglioneuroblastoma (GNB)
Synonyms
Schwannian stroma-poor neuroblastic tumor (neuroblastoma)
Schwannian stroma-rich neuroblastic tumor (ganglioneuroblastoma)
Definitions
Malignant tumor derived from primordial neural crest cells
On maturational spectrum of neuroblastic tumors
NB is least differentiated
GNB is moderately differentiated
Ganglioneuroma (GN) is well-differentiated, benign
ETIOLOGY/PATHOGENESIS
Developmental Anomaly
Derived from primordial neural crest cells
These cells migrate from spinal cord to adrenal medulla and sympathetic ganglia
CLINICAL ISSUES
Epidemiology
Incidence
About 1 in 10,000 children
3rd most common malignant tumor in children
Most common extracranial solid tumor in children
Usually sporadic
Some autosomal dominant familial cases have been seen
Screening not recommended
Age
Half of patients diagnosed by age 2 years
90% diagnosed by age 5 years
About 1/4 are congenital, with some detected prenatally on ultrasound
Gender
Slight male predominance
Ethnicity
Less common in African-Americans
Site
Follows distribution of sympathetic ganglia
Paramidline from base of skull to pelvis
Most common in abdomen and retroperitoneum
Adrenal medulla
Dorsal root ganglia
Metastases
Bone
Lymph nodes
Liver
Skin
Presentation
Depends on age of patient, location of tumor, and associated clinical syndromes
Most have nonspecific symptoms
Fever, weight loss, diarrhea, anemia, hypertension
Fetuses may have hydrops
Palpable mass in about half
About 2/3 have metastases on presentation
“Blueberry muffin” baby
Blue-red cutaneous masses in infants
Myoclonus-opsoclonus syndrome
Associated with good prognosis
Rapid, alternating eye movements and myoclonic movements of extremities
Resolves with tumor eradication
Other associated syndromes include
Laboratory Tests
Urine catecholamines (elevated in 95% of patients with NB)
Epinephrine
Norepinephrine
Homovanillic acid (HVA)
Vanillylmandelic acid (VMA)
VMA/HVA ratio > 1.5 is associated with better prognosis
Lactate dehydrogenase
> 1500 IU/L associated with worse clinical outcome
Ferritin
> 142 ng/mL associated with worse clinical outcome
Neuron-specific enolase (NSE)
> 100 ng/mL associated with worse clinical outcome
Natural History
1-2% will spontaneously regress
Most in children under age 1 year
NB can metastasize widely via lymphatics and vessels
Treatment
Low risk
Surgery or observation alone
Intermediate risk
Surgery and adjuvant chemotherapy
High risk
Induction chemotherapy
Delayed tumor resection
Radiation of primary site
Myeloablative chemotherapy with stem cell recovery
Prognosis
Favorable prognostic factors
Age < 1.5 years at diagnosis
Favorable histology
Stage 1, 2, or 4S
Related to location of tumor
No N-myc amplification
Hyperdiploidy
No loss of 1p
High expression of TrKA
Normal serum ferritin, NSE, and LDH
Urinary VMA/HVA ratio > 1.5
IMAGE FINDINGS
General Features
Extensive radiographic evaluation is required to determine extent of disease and identify metastatic foci
Calcifications often seen in central portion of tumor
Bone Scan
Radiolabeled metaiodobenzylguanidine (MIBG) incorporates into catecholamine-secreting cells and can detect neuroblastoma
MACROSCOPIC FEATURES
General Features
Neuroblastoma
Fine membranous capsules
Cut surface is soft, fleshy, often with hemorrhage and necrosis
Ganglioneuroblastoma
Cut surface is firm, gray-white
Nodular GNB must have grossly visible, usually hemorrhagic nodules
Intermixed GNB can look like NB or GN depending on extent of differentiation
Size
Average: 6-8 cm diameter
MICROSCOPIC PATHOLOGY
Histologic Features
Neuroblasts
Small round blue cells
Very little cytoplasm
Homer-Wright pseudorosette
Neuroblasts forming a ring around central core of cytoplasmic processes
Ganglionic differentiation
Cells enlarge
Increased eosinophilic or amphophilic cytoplasm
Nuclear chromatin pattern becomes vesicular
Must have synchronous differentiation of cytoplasm and nucleus
Neuropil
Fibrillar eosinophilic matrix
Mitotic-karyorrhectic index (MKI)
Count of cells undergoing mitosis or karyorrhexis, per 5,000 cells
Can be estimated
Low: < 100 cells per 5,000
Intermediate: 100-200 cells per 5,000
High: > 200 cells per 5,000
International Neuroblastoma Pathology Committee (INPC) Classification
a.k.a. Shimada classification
Undifferentiated NB
No ganglionic differentiation
No neuropil
No or minimal schwannian stroma
Often requires immunohistochemistry for accurate diagnosis
Poorly differentiated NB
< 5% of tumor cells showing ganglionic differentiation
Neuropil background
No or minimal schwannian stroma
Differentiating NB
> 5% of tumor cells showing ganglionic differentiation
Usually more abundant neuropil
Usually more prominent schwannian stroma
Must be < 50%
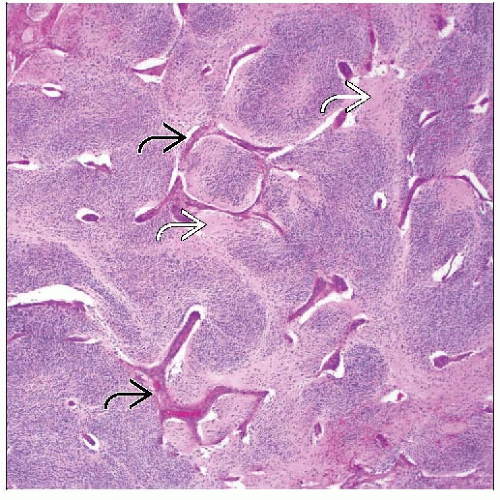
Nodular GNB
Grossly identifiable nodules will be neuroblastoma
Abrupt demarcation between stroma-poor neuroblastoma and stroma-rich component
Fibrous pseudocapsule often seen surrounding NB component
> 50% schwannian stroma
Intermixed GNB
Microscopic nests of neuroblastoma within schwannian stroma
> 50% schwannian stroma
Do not classify post-treatment resections
“Neuroblastoma with treatment effect” is sufficient
May classify metastatic disease if resection/biopsy is pre-treatment
ANCILLARY TESTS
Immunohistochemistry
Neuron-specific enolase (NSE)
Most sensitive but least specific
Is found at least focally even in very undifferentiated NBs
NB84(+) in almost all NBs
Not specific; occasionally positive in other small round cell tumors
S100 protein
Positive in schwannian stroma
Other useful positive immunostains include
Chromogranin
Synaptophysin
Protein gene product 9.5 (PGP9.5)
CD56
Cytogenetics
MYCN
Amplification is associated with worse prognosis
Usually seen in advanced disease
DNA ploidy
Near-diploidy or tetraploidy is associated with worse prognosis
Hyperdiploidy is associated with better prognosis
Loss of heterozygosity of 1p and 11q
Both associated with worse prognosis
TrkA (high-affinity nerve growth factor receptor)
Increased expression associates with better prognosis
Electron Microscopy
Wide range of cytologic differentiation
Dense core of neurosecretory granules
Found in elongated cell processes
100 nm in diameter
Dense core surrounded by clear halos and delicate outer membranes
DIFFERENTIAL DIAGNOSIS
Alveolar Rhabdomyosarcoma (ARMS)
Clinical presentations may be similar
More marked alveolar pattern except in solid variant
More pleomorphism
Cells have more abundant cytoplasm than NB
Diffuse immunoreactivity for desmin in cytoplasm
Myogenin(+) in nuclei
Characteristic t(1;13) or t(2;13) with PAX-FOXO1 fusions
Ewing Sarcoma/Primitive Neuroectodermal Tumor (PNET)
Usually in older patients
Cells have finely stippled chromatin and glycogen-filled cytoplasm
CD99 usually shows diffuse membranous immunoreactivity
Specific gene fusions, most commonly EWSR1-FLI1
Lymphoma
Lacks NSE, synaptophysin, and chromogranin
Has confirmatory lymphoid markers
CD45, CD3, CD20
TdT in lymphoblastic lymphoma
Maturing Ganglioneuroma
Differs from intermixed GNB in having single cells instead of nests of cells within schwannian stroma
SELECTED REFERENCES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree