Neck Dissection
Jatin P. Shah
Ian Ganly
Introduction
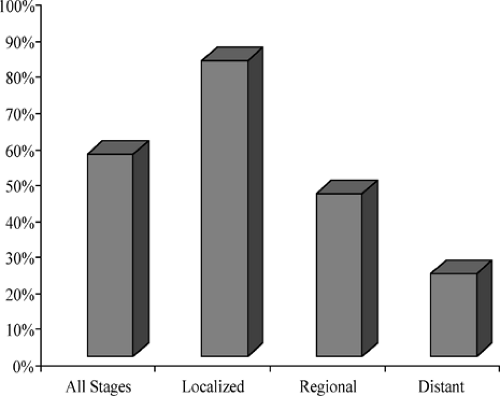
The single most important factor affecting prognosis of squamous cell carcinoma of the head and neck, the sixth most common cancer worldwide, is the status of the cervical lymph nodes. Metastases to the regional lymph nodes reduces the 5-year survival rate by 50% compared with that of patients with early stage disease (Fig. 1). The American Cancer Society has reported that 40% of patients with squamous carcinoma of the oral cavity and pharynx present with regional metastases (Fig. 2). Therefore, management of the cervical lymph nodes is an important component in the overall treatment plan for patients with squamous cell carcinoma of the head and neck.
Cervical lymph nodes are classified according to the system developed at Memorial Sloan-Kettering Cancer Center in the 1930s. This system divides the lymph nodes in the lateral aspect of the neck into five nodal levels, I through V, as shown in Fig. 3. In addition, lymph nodes in the central compartment are categorized into level VI and those in the anterior superior mediastinum as level VII. Table 1 lists the clinical and surgical landmarks used to describe these levels. Recently, level I, II, and V nodes were subclassified into levels IA and IB, IIA and IIB, and VA and VB. Level IA includes the submental lymph nodes, whereas level IB includes the submandibular lymph nodes. Level IIA includes lymph nodes below the accessory nerve, whereas IIB includes nodes above the accessory nerve. The posterior triangle has been subdivided into levels VA and VB, with the dividing line being the accessory nerve in the posterior triangle. This subdivision is based on patterns of lymph node spread from various primaries. For example, level IA lymph node spread is rare except for tumors of the lower lip and anterior floor of mouth. Recent studies have shown that, in the patients with no level IIA nodes clinically, metastatic spread to level IIB nodes is rare. Similarly, in thyroid cancer, studies have shown that metastatic spread to level VA lymph nodes is exceedingly rare.
Staging System for Metastatic Squamous Cell Carcinoma of the Neck
A uniform staging system for regional metastases to cervical lymph nodes was established by the American Joint Committee on Cancer and the International Union Against Cancer. The staging system for squamous cell carcinoma is shown in Table 2 and Fig. 4. The staging system for thyroid carcinoma is shown in Table 3. The staging system is based on both the size and the number of enlarged lymph nodes. Both these factors have important prognostic significance. The prognosis worsens with increasing N stage. However, there are other nodal factors affecting prognosis that are not included in the staging system.
Nodal Factors Affecting Prognosis
Characteristics of regional nodes that affect prognosis include the presence of pathologically positive nodes, size of the metastatic lymph node, the number of lymph nodes involved, and the location of the lymph nodes. Involvement of the lower cervical nodes (level IV) and the lower posterior triangle lymph nodes has a very poor prognosis. Another important prognostic factor is the presence of extranodal spread where the capsule of the lymph node is ruptured, resulting in invasion of the surrounding soft tissues. This increases both the incidence of regional recurrence and also distant metastases. In a clinically positive N1 neck, there is a 30% incidence of extranodal spread, whereas in the clinically positive N2a/N3 neck, extranodal spread is present in 50% to 70%. Perivascular and perineural infiltration by tumor also have a negative effect on prognosis. All these factors must be considered when planning adjuvant treatment following neck dissection.
Risk Factors for Nodal Metastasis
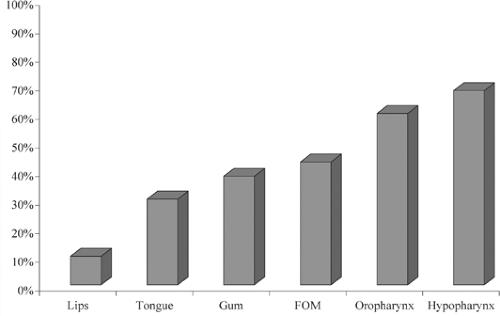
The risk for cervical node metastases is influenced by characteristics of the primary tumor such as location, size, and histology. As a general rule, the risk for lymph node metastases increases for more posteriorly located tumors, such as those of the oropharynx and hypopharynx compared to lips and oral cavity (Fig. 5). For example, oropharyngeal cancers are at higher risk than oral cavity tumors. Lesions of the tonsil
and base of tongue have a very high incidence of nodal metastases. Tumors of the hypopharynx universally have lymph node metastases. The risk of nodal metastases is higher for tumors of the supraglottic larynx compared with the glottic larynx because of the relative absence of lymphatic vessels in the glottic larynx. The greater the T size of the primary tumor, the greater the probability of having lymph node metastases. For example, T1, T2, and T3 tongue cancers have an incidence of metastatic disease to the neck of 30%, 50%, and 70%, respectively. Pathologic features such as endophytic versus exophytic tumors, poorer degree of differentiation, depth of invasion, vascular invasion, and perineural invasion also determine the risk of cervical metastases.
and base of tongue have a very high incidence of nodal metastases. Tumors of the hypopharynx universally have lymph node metastases. The risk of nodal metastases is higher for tumors of the supraglottic larynx compared with the glottic larynx because of the relative absence of lymphatic vessels in the glottic larynx. The greater the T size of the primary tumor, the greater the probability of having lymph node metastases. For example, T1, T2, and T3 tongue cancers have an incidence of metastatic disease to the neck of 30%, 50%, and 70%, respectively. Pathologic features such as endophytic versus exophytic tumors, poorer degree of differentiation, depth of invasion, vascular invasion, and perineural invasion also determine the risk of cervical metastases.
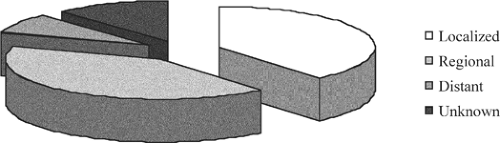 Fig. 2. Distribution of patients with squamous cell carcinoma of the head and neck in relation to extent of disease at the time of initial diagnosis. |
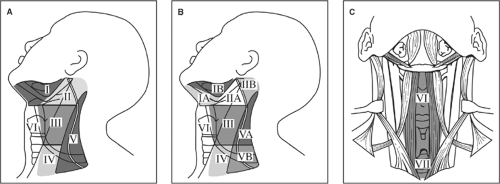 Fig. 3. Memorial Sloan-Kettering Cancer Center leveling system of cervical lymph nodes (A); current modification of leveling system (B); and levels VI and VII (C). |
Table 1 Clinical and Surgical Landmarks for Neck Node Levels | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 2 Staging System of Regional Lymph Nodes (N Stage) for Squamous Cell Carcinoma of the Upper Aerodigestive Tract Excluding Nasopharynx | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
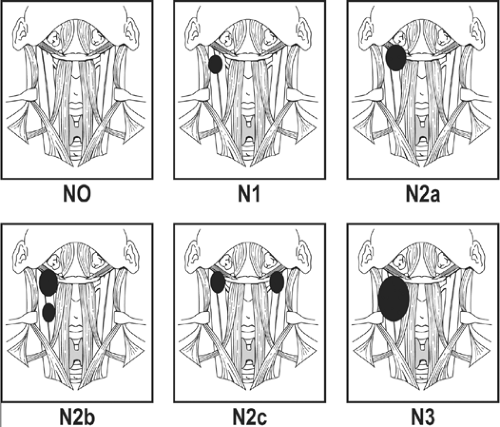 Fig. 4. Staging system of regional lymph nodes (N stage) for squamous cell carcinoma of the upper aerodigestive tract, excluding the nasopharynx. |
Table 3 Staging System of Regional Lymph Nodes (N Stage) for Thyroid Carcinoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Patterns of Nodal Metastases
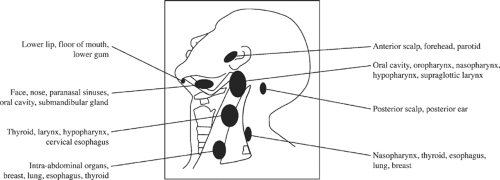
The location of metastases is mainly determined by the location of the primary site. Figure 6 illustrates the nodes typically affected by primary tumor location. Cancers of the oral cavity typically spread first to the nodes in levels I to III, whereas cancers of the oropharynx, hypopharynx, and larynx spread first to the nodes in levels II to IV. This observation is based on the philosophy that nodal spread of cancer proceeds in an orderly and predictable fashion as determined by the lymphatic drainage pattern in the neck. In 1972, Lindberg, from the M.D. Anderson Cancer Center, was the first to report that the lymph node groups most frequently involved in cancer of the oral cavity were level II/III, and in patients with cancer of the floor of mouth, oral tongue, and buccal mucosa, the nodes most frequently involved were located in the submandibular triangle (level IB). Lindberg also reported that cancers can metastasize to both sides of the neck and can skip the submandibular and jugulodigastric nodes metastasizing first to the midjugular nodes (level III).
The patterns of nodal metastasis were later well described by Shah, from Memorial Sloan-Kettering Cancer Center, in 1990. To determine lymph node levels at risk from a particular primary site, Shah analyzed pathology specimens from 1,119 classic radical neck dissections (RNDs) for squamous cell carcinoma of the upper aerodigestive tract. This consisted of 343 RNDs for the clinically negative neck (N0) and 776 RNDs for the clinically positive neck. From these studies, the incidence of pathologically positive neck specimens was 82% for the clinically positive neck and 33% for the clinically negative neck. Tables 4 and 5 show the percentage of patients with pathologically positive nodes at each level for clinically positive and clinically negative disease.
In the clinically positive neck setting (Table 4), patients with primary oral cavity tumors had the majority of positive nodes in levels I to III; levels IV and V were involved in 20% and 4% of specimens, respectively. In patients with primary oropharyngeal tumors, the majority of positive nodes were in levels II to IV; levels I and V were involved in 17% and 11% of specimens, respectively. In patients with hypopharyngeal tumors, most positive nodes were in levels II to IV; levels I and V were involved in 10% and 11% of specimens, respectively. In patients with primary tumors of the larynx, most positive nodes were in levels II to IV; levels I and V were involved in 8% and 5%, respectively.
In the clinically negative neck setting (Table 5), patients with primary oral cavity tumors had the majority of positive nodes in levels I to III; levels IV and V were involved in 9% and 2% of specimens, respectively. In patients with primary oropharyngeal tumors, the majority of positive nodes were in levels II to IV; levels I and V were involved in 7%. In patients with hypopharyngeal tumors, most positive nodes were in levels II to IV; levels I and V were not involved. In patients with primary tumors of the larynx, most positive nodes were in levels II to IV; levels I and V were involved in 14% and 7%, respectively.
The question of level V metastases was addressed in a separate study on 1,277 RNDs by Davidson et al. in 1993. Metastases were found in 40 (3%) patients. Level V metastases were highest in patients with hypopharyngeal and oropharyngeal primary sites (7% and 6%, respectively). Only 3 out of 40 patients with a clinically negative neck had a positive level V lymph node. Therefore, the incidence of level V metastases is small and extremely unlikely in the clinically negative neck setting.
Table 4 Percentage of Positive Lymph Nodes in the CN+ Neck | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
History
The importance of the regional cervical lymphatics in oral cavity cancer was noted by Chelius in 1847 who commented, “the neighboring lymphatics become hard and painful” and “once the growth in the mouth has spread to the submaxillary gland, complete removal of the disease is impossible.” In 1847, Warren described the attempted removal of cancer in the neck through an incision from the masseter muscle to the clavicle, although this must have been an unplanned procedure, not based on any anatomical considerations. Kocher in 1880 described the removal of the tongue for cancer through the submaxillary triangle, first removing the lymphatics and submaxillary and sublingual salivary glands. He later proposed that the cervical lymphatics should be removed more widely and described the “Kocher” incision, a Y-shaped incision with the long arm running from the mastoid tip down the anterior border of sternocleidomastoid muscle to the omohyoid muscle, and the short limb running at right angles to the submental region. Later in 1885, Butlin described the removal of cervical lymph nodes for tongue cancer and even discussed the prophylactic removal of these “glands” for tongue cancer.
Solis-Cohen of Philadelphia, America’s first head and neck surgeon, later advocated the removal of cervical lymph nodes during total laryngectomy. However, most of the credit for neck dissection as a curative operation for cervical metastases belongs to George Washington Crile from the Cleveland Clinic. In 1900, he performed different types of neck dissections and subsequently described the classic operation of RND in his seminal article of 1905 published in the Transactions of the Southern Surgical and Gynecological Association. This operation is now considered to be the basic neck dissection and all other procedures are considered to be modifications. George Crile later described his experience with 132 operations of RND in 1906. In this operation, all lymphatic tissues
in the lateral neck from levels I to V are systematically removed in conjunction with the sternocleidomastoid muscle, internal jugular vein, the spinal accessory nerve, and the submandibular salivary gland. The operation was popularized by Hayes Martin from Memorial Sloan-Kettering Cancer Center, who described the stepwise procedure of RND in his classic article in 1951. However, this operation is not without morbidity, as it results in a cosmetic deformity and dysfunction of shoulder movement.
in the lateral neck from levels I to V are systematically removed in conjunction with the sternocleidomastoid muscle, internal jugular vein, the spinal accessory nerve, and the submandibular salivary gland. The operation was popularized by Hayes Martin from Memorial Sloan-Kettering Cancer Center, who described the stepwise procedure of RND in his classic article in 1951. However, this operation is not without morbidity, as it results in a cosmetic deformity and dysfunction of shoulder movement.
Table 5 Percentage of Positive Lymph Nodes in the CN0 Neck | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
This led to the development of modified/functional neck dissections. Oswaldo Suarez from Argentina was the first to describe functional neck dissection in 1963, now called modified radical neck dissection (MRND). He described the removal of all five lymph node levels in the neck while preserving the spinal accessory nerve, sternocleidomastoid muscle, and internal jugular vein to limit any functional disability in the shoulder. However, his publications were in Spanish and therefore the technique was not popularized until Ettore Bocca, who learned the technique from Suarez, and published it in the English literature in 1967. Selective removal of regional nodal groups based on predictable patterns of lymph node spread were later popularized by Ballantyne from M.D. Anderson Cancer Center. In 1985, Byers from M.D. Anderson Cancer Center used the terms “anterior” and “supraomohyoid” neck dissection to describe the selective neck dissection procedure for cancers of the oral cavity and pharynx. These neck dissections were described for use in patients with clinically negative neck and were based on the philosophy that nodal spread of cancer proceeded in an orderly and predictable fashion. Unfortunately, the terms “modified neck dissection,” “functional neck dissection,” and “selective neck dissection” led to considerable confusion. Therefore, in 1991 the American Academy of Otolaryngology-Head and Neck Surgery published an article classifying neck dissections into comprehensive and selective. This was later updated in 2002. Structures removed and indications for these different types of neck dissection are shown in Table 6.
Comprehensive Neck Dissection
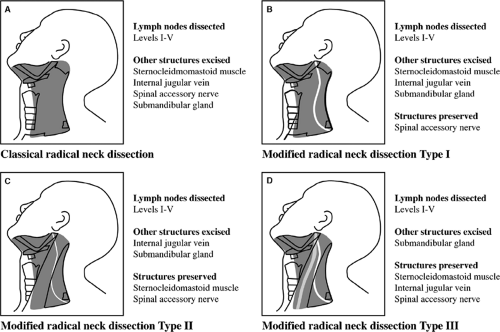
Comprehensive neck dissections involve the removal of all lymphatic tissues in the lateral neck (levels I to V) and are generally carried out for the clinically positive neck (N+). They can be classified into RND and MRND (Fig. 7), depending on what other structures are excised. RND involves the removal of lymph nodes in levels I to V, and also the sternocleidomastoid muscle, internal jugular vein, spinal accessory nerve, and submandibular salivary gland. MRND is divided into type I, II, or III, depending on the structures that are preserved. Type I MRND involves preservation of one structure, the spinal accessory nerve. Type II involves preservation of two structures: the spinal accessory nerve and the sternocleidomastoid muscle. Type III involves preservation of the spinal accessory nerve, internal jugular vein, and the sternocleidomastoid muscle. Type I MRND is the most commonly employed neck dissection for squamous cell carcinoma of the upper aerodigestive tract with clinically positive neck disease. Type III MRND is most commonly employed for metastatic-differentiated carcinoma of the thyroid.
Table 6 Classification of Different Types of Neck Dissection with Clinical Indications | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
Selective Neck Dissection
Selective neck dissection spares all nonlymphatic tissues, including the sternocleidomastoid muscle, internal jugular vein, and spinal accessory nerve. However, it does not
remove all the lymphatic tissues on the involved side of the neck as does a comprehensive neck dissection, but rather uses the selective removal of nodal regions at risk. This is determined by the predictive pattern of metastases based on the location of the primary tumor. It is based on the clinical observation that squamous cell carcinoma of the upper aerodigestive tract metastasizes in a predictable and sequential pattern. Selective neck dissections are therefore generally carried out for the clinically negative neck (N0), where there is at least a 15% to 20% risk of occult metastatic disease. Additional indications may be situations in which surgical access to the primary extends to lymph node groups at risk of metastases. More controversially, it may be used for nodal metastases confined to the first-echelon nodes (usually N1) when the primary is being treated by surgery. However, it is important to point out that the neck requires postoperative radiation therapy in this setting, as reported by Byers, Pellitteri et al., Spiro et al., and Traynor et al.
remove all the lymphatic tissues on the involved side of the neck as does a comprehensive neck dissection, but rather uses the selective removal of nodal regions at risk. This is determined by the predictive pattern of metastases based on the location of the primary tumor. It is based on the clinical observation that squamous cell carcinoma of the upper aerodigestive tract metastasizes in a predictable and sequential pattern. Selective neck dissections are therefore generally carried out for the clinically negative neck (N0), where there is at least a 15% to 20% risk of occult metastatic disease. Additional indications may be situations in which surgical access to the primary extends to lymph node groups at risk of metastases. More controversially, it may be used for nodal metastases confined to the first-echelon nodes (usually N1) when the primary is being treated by surgery. However, it is important to point out that the neck requires postoperative radiation therapy in this setting, as reported by Byers, Pellitteri et al., Spiro et al., and Traynor et al.
Common selective neck dissections are shown in Fig. 8. These include the supraomohyoid neck dissection (SOHND), in which lymph nodes in levels I to III and the submandibular salivary gland are removed (Fig. 8A); the extended SOHND, in which lymph nodes in levels I to IV and the submandibular gland are removed (Fig. 8B); the anterolateral neck dissection (LND), in which lymph nodes in levels II to IV are removed (Fig. 8C); posterolateral neck dissection (PLND), in which lymph nodes in levels II to V and also the suboccipital and retroauricular lymph nodes are removed (Fig. 8D); and central or anterior compartment neck dissection, in which lymph nodes at level VI in the prelaryngeal, pretracheal, and paratracheal regions are removed (Fig. 8E).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree