Nasal drug delivery
Gary P. Martin and Alison B. Lansley
Chapter contents
Key points
Introduction
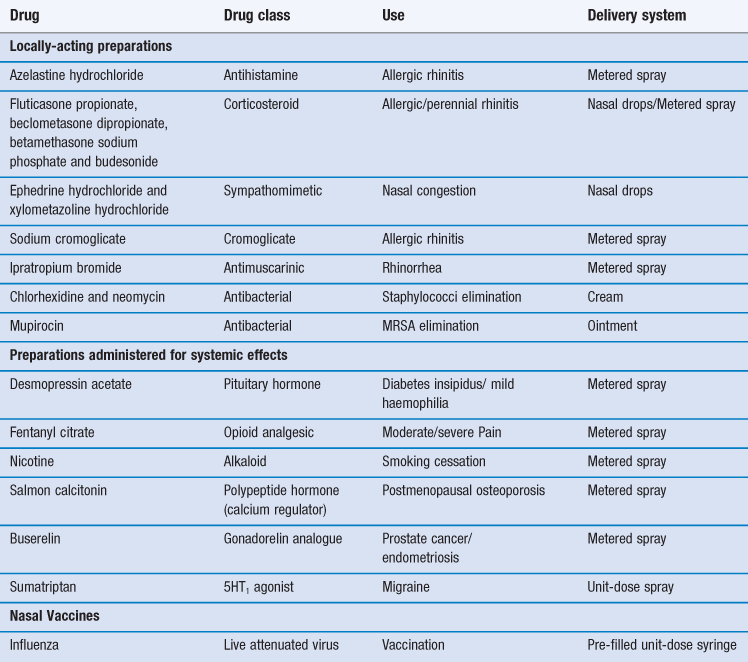
The most common reason for introducing a drug into the nasal cavity is to provide a convenient and accessible route for rapidly and efficiently managing the localized symptoms associated with allergic rhinitis, nasal congestion and nasal infection. Drugs applied topically for such purposes include antihistamines, corticosteroids, sodium cromoglicate, sympathomimetics and antiseptics/antibiotics (Table 38.1). These drugs are administered either in liquid form (from a spray or as drops) or as creams/ointments.
The intranasal route has also been exploited for the delivery of drugs to the systemic circulation (Table 38.1). There are several possible reasons for pharmaceutical companies to consider employing this route for marketed medicines rather than the much more popular (and preferred) oral route. These include:
The nasal cavity has also been utilized, or proposed, as a portal for the delivery of vaccines, particularly for infections associated with the respiratory tract such as influenza and possibly, eventually, tuberculosis. The presentation of an antigen, in combination with an acceptable adjuvant to the nasal-associated lymphoid tissue (NALT) can promote both cellular and humoral responses. However human vaccination need not be restricted to airways infections and systemic immune responses are demonstrable after introduction of appropriate antigens via this route. Indeed, intranasal vaccination has been studied with a view to combating noroviruses, the measles and herpes viruses, diphtheria and tetanus microorganisms. An intranasal vaccine containing live attenuated influenza vaccine has been marketed (Table 38.1).
Currently, there is much research interest aimed at establishing whether the olfactory region, positioned in the upper reaches of the nasal cavity (Fig. 38.1), offers a potential means, in humans, of circumventing the obstacles imposed by the blood-brain barrier to the access of many drugs from the bloodstream to the brain. This region contains a direct physiological link between the environment and the central nervous system (CNS). Clearly, if such a route could be established conclusively as being viable for the delivery of therapeutic quantities of drugs in humans, then this would have great potential in treating conditions such as: Alzheimer’s disease, brain tumours, epilepsy, pain and sleep disorders. However, although there are a number of studies that suggest that drugs may be absorbed from the olfactory region, the true significance of many findings are confounded by the use of animal models with the data often being extrapolated uncritically to humans.
So as to appreciate fully the potential of the nasal cavity for drug delivery, it is pertinent to review the relevant anatomy and physiology, consider in more detail the applications of utilising the route, review the physicochemical properties of administered drugs and factors that might affect the choice of formulation and discuss the devices that can be employed to deliver nasal medicines.
Anatomy and physiology
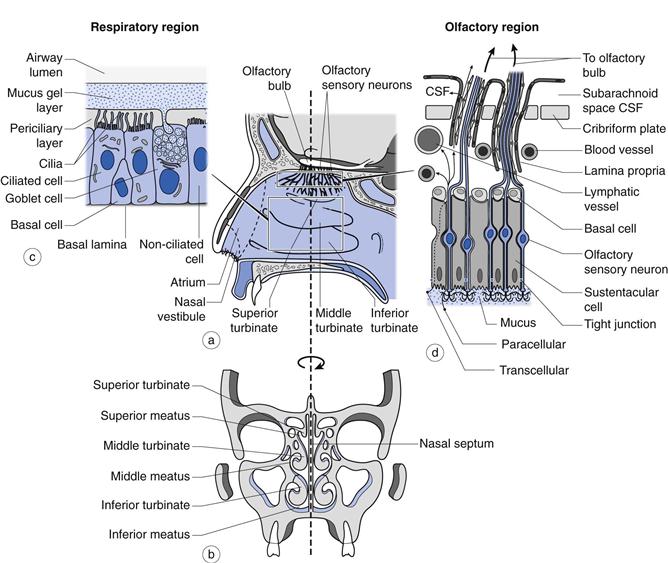
The nasal cavity is 120–140 mm from the nostrils to the nasopharynx (Fig. 38.1) and is divided in two by the nasal septum. The total surface area of both cavities is about 160 cm2 and the total volume is about 15 mL. The first part of the nasal cavity (termed the nasal vestibule) contains the narrowest part of the nasal cavity with a cross-sectional area of 30 mm2 on each side. The lining of the vestibule changes from skin at the entrance, to a stratified squamous epithelium which extends over the anterior third of the entire nasal cavity. The nasal vestibule contains vibrissae (hairs) which filter out inhaled particles with an aerodynamic particle size greater than approximately 10 µm. Progression through the nasal cavity leads to the turbinate region. The turbinates are convoluted projections from the nasal septum which are lined with a pseudostratified columnar epithelium (80–90% of the total surface area of the nasal epithelium in man) composed of mucus-secreting goblet cells, ciliated and non-ciliated cells and basal cells (Fig. 38.1). The apical surfaces of the ciliated and non-ciliated cells are covered with non-motile microvilli, which serve to increase the surface area of the epithelial cells. There are also approximately 100 motile cilia on each ciliated cell which are responsible for mucus transport. Serous and seromucous glands also contribute to nasal secretions. As air moves through the turbinate region via the meatuses (Fig. 38.1), the low rate of airflow in combination with the turbulence created by the shape of the turbinates encourages the air to make contact with the highly vascularized walls, enabling it to be warmed and humidified. Particulates (5–10 µm) within the airstream, such as dust, pollen, microorganisms and pollutants have the potential to deposit on the viscoelastic mucous gel lining the turbinate walls. The cilia, beating within the periciliary fluid, engage with the underside of the mucus and propel the gel and the deposited particles to the nasopharynx, where they are either swallowed or expectorated. This process is termed mucociliary clearance and is able to clear mucus at a rate of about 7 mm min−1. About 20% of the inspired air is directed to the top of the turbinates where the olfactory region is located (Fig. 38.1). This is an area approximately 12.5 cm2 (~ 8% of the total surface area of the nasal epithelium in man) of non-ciliated pseudostratified columnar epithelium traversed by 6–10 million olfactory sensory neurons which pass from the nasal cavity, between the epithelial (sustentacular) cells and through the cribriform plate to the olfactory bulb of the brain.
Drug delivery
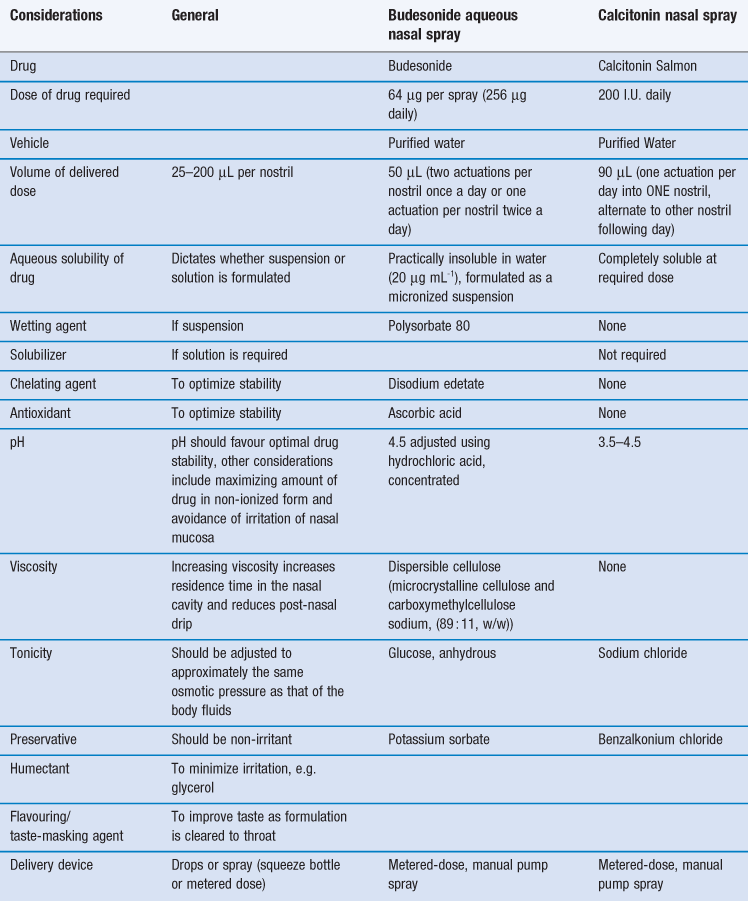
Certain constraints are imposed upon formulating preparations for the nasal route and two case studies, one a locally-acting drug (budesonide) and a second systemically-acting peptide drug (calcitonin) are given in Table 38.2.
As with the formulation of any medicine, the information garnered from pre-formulation studies (Chapter 23) is an essential prerequisite in the design of an intranasally delivered medicine. The solubility (Chapter 2) of the drug to be administered is a key determinant in the final formulation. The restricted volume that can be applied to the nasal cavity also impacts upon the nature of the resultant formulation. Generally, the premise of presenting the drug in the simplest formulation, containing the fewest excipients possible to ensure a stable medicine with an adequate shelf-life is the course that should be followed in the development process. Currently, delivery devices are usually metered-dose manual pump sprays, since these are cheap, robust and reliable but more sophisticated systems are now under development, as discussed below.
Local delivery
For conditions affecting the nose, it is logical to deliver the drug directly to its site of action. This permits the rapid relief of symptoms with a much lower dose of drug than would be necessary if it were delivered by the oral route, and reduces the chance of systemic side effects. For example, this is particularly pertinent when delivering corticosteroids to reduce local inflammation of the nasal mucosa and sinuses, without causing pituitary-adrenal suppression, or alternatively when using localized antihistamine therapy without inducing drowsiness.
Systemic delivery
The rationale for the use of the nasal cavity for systemic delivery includes its accessibility, avoidance of pre-systemic metabolism and potential to provide a rapid onset of action. Its use for peptides (Tables 38.1 and 38.2) has been successful since, although only a very low percentage of administered drug is absorbed (i.e. low bioavailability), the attained plasma levels are sufficient for therapeutic efficacy. Many of the marketed nasally administered peptides have wide therapeutic windows. Therefore, providing the minimum therapeutic level is exceeded in the bloodstream, a large variability in the final attained plasma level can be tolerated, without systemic toxicity becoming manifest. Any potential localized toxicity can be minimized in chronic administration by alternating nostrils when daily dosing (Table 38.2). Intranasal delivery can also be useful in emergency situations, such as in the treatment of opioid overdose (using naloxone) or in the treatment of intractable childhood seizures (using benzodiazepines). Drug delivery via this route is also well-suited to drugs that, when administered orally, cause emesis e.g. galantamine used to treat dementia.
Anatomical and physiological factors affecting intranasal systemic delivery
For a drug molecule to enter the systemic circulation it must first be absorbed across the nasal epithelium.
This may occur via the mechanisms of passive diffusion via the transcellular or paracellular routes (Chapter 19). The transcellular pathway is the principal route of absorption for lipophilic molecules, while small, hydrophilic molecules diffuse between the epithelial cells (paracellularly) via the tight junctions which are dynamic structures responsible for the integrity of the nasal epithelium. This latter pathway avoids the need for the drug molecules to partition into and out of the lipophilic membrane of the epithelial cells, but imposes a size restriction of between 0.39–0.84 nm. Transcellular absorption can also occur via endocytosis, the route exploited by large hydrophilic molecules (>1 kDa), and via active transport mechanisms where drug molecules with a similar structure to a natural substrate can interact with a carrier protein to cross the epithelial cells.
Since most drug absorption takes place by passive diffusion, the relatively large surface area of the nasal cavity and its rich blood supply (which helps to maintain the concentration gradient across the epithelium) aid this process. Working against these positive attributes of the nasal cavity are the barriers presented by mucus and the epithelium itself and the nasal clearance mechanisms including mucociliary clearance and metabolism. The advantages and disadvantages of the nasal cavity for systemic drug delivery are summarized in Table 38.3.
Table 38.3
Advantages and disadvantages of intranasal drug delivery for systemic activity
| Advantages | Disadvantages |
| Large surface area for absorption (approximately 160 cm2) | Limited to small delivery volumes (25–200 µL) therefore require potent drugs |
| Good blood supply and lymphatic system | Mucociliary clearance, mucus barrier |
| Avoids hepatic first-pass metabolism | Enzymatic activity (pseudo first-pass effect) |
| Epithelium is permeable to small, lipophilic drug molecules; rapid absorption and onset of action | Low epithelial permeability for hydrophilic drugs; require absorption enhancers and large doses |
| Non-invasive, so minimal infection risk during application and low risk of disease transmission (unlike parenteral route) | |
| Easy to self-administer and adjust dose |
Mucociliary clearance
The main drug absorption site is the respiratory epithelium of the nasal turbinates, which is where mucociliary clearance dominates. Drug deposited anterior to this region will remain in the nasal cavity for longer than drug deposited in the turbinates, but absorption from this site is less. Once drug particles (if formulated as a suspension) or molecules (if in solution) find their way on to the mucociliary ‘conveyor belt’ they will be cleared from the nasal cavity and therefore have a limited contact time with the absorption site. For drugs which are in solution and rapidly absorbed (lipophilic, low molecular weight) the limited contact time is likely to be well in excess of that required for complete absorption. However, for drug particles needing time to dissolve prior to absorption, and for polar drug molecules with a low rate of absorption once in solution, the rate of mucociliary clearance is likely to play a significant role in limiting the extent of absorption.
Mucus barrier
The nasal mucosa is protected from the external environment by a layer of mucus. In the nasal cavity this exists as a gel phase which is approximately 1–10 µm thick and found above a watery, sol phase surrounding the cilia (periciliary layer) which is about 7 µm deep (Fig. 38.1). Mucus is secreted continuously by the goblet cells and submucosal glands. Normal mucus is 97% water and 3% solids; with the latter comprising: i) mucins (about 30% of the solid content), ii) non-mucin proteins (e.g. albumin, immunoglobulins, lysozyme and lactoferrin), iii) inorganic salts and iv) lipids. Mucins are extremely large glycoproteins (up to 3 × 106 daltons per monomer) with protein regions rich in serine and threonine which are linked, by their hydroxyl side groups, to sugar chains (O-glycosylation). They are anionic (negatively-charged) because most of their terminal sugars contain carboxyl or sulphate groups. These glycosylated (sugar-rich) regions are separated by regions of non-glycosylated, ‘naked’ protein, rich in cysteine residues, which are believed to form globular domains stabilized by disulfide bonds. These ‘naked’ domains are the most hydrophobic regions of mucins and probably adsorb significant amounts of lipids. They are also the most antigenic sites on mucins. Entanglement of mucin polymers leads to the formation of a mucous gel and the generation of a mesh which is stablized by non-covalent calcium-dependent cross-linking of adjacent polymers. The sugar side chains bind large amounts of water allowing the mucus to act as a lubricant and a reservoir for the periciliary fluid within which the cilia beat. Mucus is a viscoelastic gel with properties of both a deformable solid (elasticity) and a viscous fluid (Chapter 6). Cilia can only transport mucus of the appropriate viscoelasticity and this is controlled by the level of mucus hydration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree