Myoepithelial Neoplasms
Myoepithelial cells comprise part of the normal microscopic anatomy of mammary lobules and ducts (1). They participate in many benign proliferative processes, most notably sclerosing adenosis and papillary proliferative lesions of ducts (2,3). Benign tumors composed of myoepithelial cells are termed myoepitheliomas. If epithelial and myoepithelial cells participate in the proliferation, the term adenomyoepithelioma is appropriate. Malignant neoplasms formed by myoepithelial cells may have an epithelial phenotype (myoepithelial carcinoma), a myoid phenotype (leiomyosarcoma), or both appearances (malignant adenomyoepithelioma) (4,5,6).
The diagnosis of myoepithelial or adenomyoepithelial neoplasms in needle core biopsy and fine needle aspiration (7) samples of the breast can be a very challenging problem, especially for pathologists who have little experience with the histologic spectrum of these uncommon tumors. Attention is drawn especially to benign myoepithelial tumors with a predominately epithelial appearance, benign adenomyoepitheliomas in which glandular elements are dispersed amidst spindly myoepithelial cells, and tumors that qualify as “pleomorphic adenomas.” When seen out of the context of a complete histologic section, needle core biopsy and fine needle aspiration samples from any of these benign lesions can be mistaken for invasive carcinoma (7). If the possibility of a myoepithelial or adenomyoepithelial neoplasm is considered in this situation, selected appropriate immunostains will almost always resolve the diagnosis in the needle core biopsy sample.
ADENOMYOEPITHELIOMA
All patients have been women ranging in ages 26 to 82 (average about 60 years) who presented with a solitary unilateral painless mass. Nipple discharge, pain, and tenderness are infrequent. The mammographic findings may be interpreted as suspicious in some patients (6). Nonpalpable adenomyoepitheliomas measuring 2 cm or less have been detected as well-circumscribed mass lesions by sonography (8) or by mammography (9). Calcifications are sometimes, but not always, present. One malignant adenomyoepithelioma appeared to be cystic on mammography (10).
The majority of adenomyoepitheliomas are variants of intraductal papilloma, but a small number of these tumors appear to arise from a lobular proliferation. Adenomyoepithelioma is closely related to ductal adenoma (11,12) and pleomorphic adenoma (mixed tumor) (13,14). Foci of adenomyoepithelioma can frequently be detected in tubular adenomas and in mammary pleomorphic adenomas. As is the case with sclerosing papillary tumors, generally, the needle core biopsy sample obtained from an adenomyoepithelioma can be misinterpreted as invasive carcinoma as reported by Zhang et al. (7).
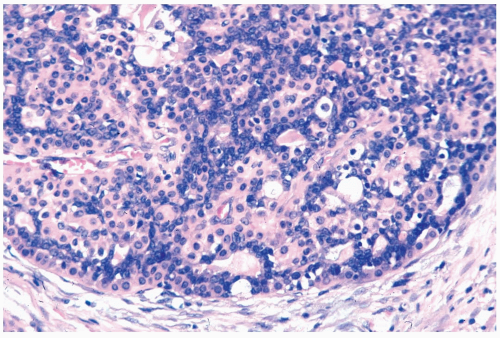
Microscopically, adenomyoepitheliomas are circumscribed and composed of aggregated nodules (Fig. 5.1). Some nodules consist of a compact proliferation of epithelial and myoepithelial cells, but most lesions have one or more nodules in which there is at least a focal papillary growth pattern. Sometimes the papillary intraductal component extends into ducts outside the gross tumorous lesion. This characteristic may be responsible for local recurrence after a seemingly adequate excision.
The basic microscopic structural unit of the adenomyoepithelioma is a small glandular lumen encompassed by cuboidal epithelial cells. Surrounding the glands are polygonal or spindle-shaped myoepithelial cells with eosinophilic or clear cytoplasm and a basement membrane (Fig. 5.2). The most common microscopic pattern, sometimes referred to as the tubular type of adenomyoepithelioma, is a proliferation of tubular glandular elements separated by islands and bands of polygonal myoepithelial cells that have clear cytoplasm. In some lesions myoepithelial cells that proliferate between glands in broad bands and trabeculae are separated by strands of basement membrane and stroma (Figs. 5.3 and 5.4). The
contrast between the dark staining cytoplasm of glandular cells and the pale cytoplasm of myoepithelial cells is striking. Fragments of adenomyoepithelioma in needle core biopsy specimens can be mistaken for infiltrating carcinoma (Fig. 5.5).
contrast between the dark staining cytoplasm of glandular cells and the pale cytoplasm of myoepithelial cells is striking. Fragments of adenomyoepithelioma in needle core biopsy specimens can be mistaken for infiltrating carcinoma (Fig. 5.5).
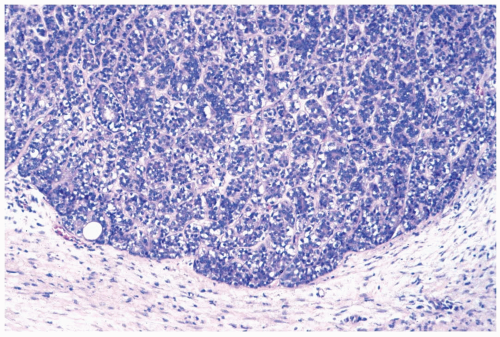 Figure 5.1 Adenomyoepithelioma. The lesion has a well-cir-cumscribed border. Darkly stained epithelial cells and myoepithelial cells with clear cytoplasm are apparent. |
Apocrine metaplasia may be encountered in the glandular epithelium, particularly in papillary areas, and it can be cytologically atypical. Foci of sebaceous and squamous metaplasia are variably present (Fig. 5.6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree