Myeloid/Monocytic Sarcoma
Carlos E. Bueso-Ramos, MD, PhD
Key Facts
Clinical Issues
Myeloid/monocytic sarcoma (MS) is tumor mass
Can develop de novo (27%), concurrently with, or after diagnosis of AML
Can arise as blastic phase of MDS, MPN, or MDS/MPN
Any anatomic site of body can be involved
Most common: Skin, lymph node
MS effaces tissue architecture at extramedullary sites
Microscopic Pathology
Blasts often infiltrate tissues in single file pattern
Blasts have thin nuclear membranes, “dusty” chromatin, high mitotic rate
Most cases of MS are composed of granulocytes with variable differentiation
Subset of cases of MS exhibit myelomonocytic or monocytic differentiation
MS cases composed predominantly of megakaryoblasts or erythroblasts are rare
Often a manifestation of MS arising from MPN
Ancillary Tests
MS expresses an array of myeloid-associated antigens
CD68/KP-1(+), lysozyme(+), CD43(+) > 95%
Myeloperoxidase(+): ˜ 90%; CD117(+): ˜ 80%
Conventional cytogenetics is useful for prognosis and classification
Chromosomal aberrations are detected in ˜ 50%
FISH shows clonal abnormalities in ˜ 50%
Molecular genetics
NPM1 mutations ˜ 15%; FLT3 mutations ˜ 15%
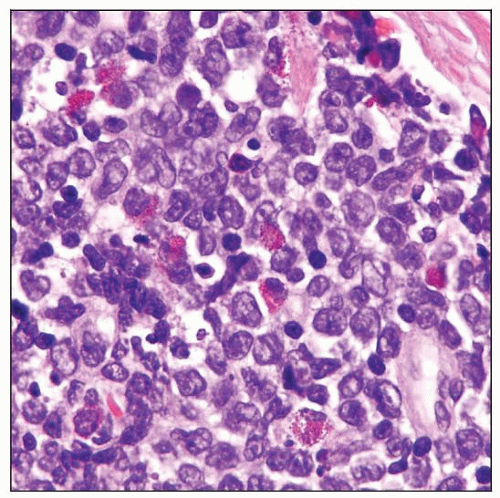 Myeloid (granulocytic) sarcoma. The neoplastic cells are immature but show evidence of differentiation, as shown by the presence of eosinophilic myelocytes and eosinophils. |
TERMINOLOGY
Abbreviations
Myeloid/monocytic sarcoma (MS)
Synonyms
Granulocytic sarcoma
Extramedullary myeloid cell tumor
Chloroma, myeloblastoma
Definitions
Tumor mass consisting of immature myeloid cells (blasts) presenting at extramedullary site
MS is equivalent to a diagnosis of acute myeloid leukemia (AML)
ETIOLOGY/PATHOGENESIS
Developmental Anomaly
Patients with certain inherited diseases have increased risk of AML/MS
Fanconi anemia, Down syndrome, Klinefelter syndrome, ataxia-telangiectasia, neurofibromatosis
Environmental Exposure
Ionizing radiation
Chemotherapy with cytotoxic agents and topoisomerase II inhibitors
Chemicals, such as benzene, pesticides, and herbicides
Cigarette smoking
CLINICAL ISSUES
Epidemiology
Age
Median age: 56 years (very wide age range)
Gender
Male to female ratio: 1.2 to 1
Site
Almost any anatomic site of body can be involved by MS
Most common sites of MS at time of initial diagnosis are
Skin (28-43%)
Lymph node (16-22%)
Central nervous system (CNS) (3-9%)
Testis (7%)
Intestines (7%)
Bladder (4%)
Gynecologic tract (4%)
Pleura and chest wall (4%)
Bone (3%)
Multiple anatomical sites (< 10% of cases)
Presentation
MS can develop de novo (27%), concurrently with, or after diagnosis of
AML
Myeloproliferative neoplasm (MPN)
Myelodysplastic syndrome (MDS)
MDS/MPN, e.g., chronic myelomonocytic leukemia (CMML)
In de novo cases, MS can precede AML by months or years
˜ 30-40% of patients with MS have simultaneous evidence of AML
MS can be manifestation of relapse in patient with previous AML
˜ 40% of patients with MS have history of AML, MPN, MDS, MDS/MPN, or mastocytosis
5-10% of patients with MS have history of therapy of nonhematopoietic tumor
These MS cases may be therapy-related
Rare MS patients have history of acute lymphoblastic leukemia
Misdiagnosis of MS is common; correct diagnosis requires
High index of suspicion
Ancillary studies
Monoblastic sarcoma commonly involves skin (˜ 50% of cases)
Cutaneous disease is common in terminal phase of CMML
Treatment
De novo MS is sensitive to radiotherapy &/or chemotherapy with possible prolonged survival
Patients with MS should undergo high-dose anti-AML therapies as front-line approach
Prognosis
Event-free survival is longer for patients with MS than for patients with AML
Underlying MDS, MPN, MDS/MPN, or AML may be negative prognostic factor
IMAGE FINDINGS
Radiographic Findings
MS shows increased FDG uptake with mean SUVmax and SUVavg of 5.1 and 3.4, respectively
Combined FDG PET/CT is more accurate for detecting lesions than FDG PET or CT alone
FDG PET/CT appears to be promising diagnostic and monitoring tool in management of patients with MS
MACROSCOPIC FEATURES
Gross Pathology
MS with granulocytic differentiation is designated as chloroma because tumors have green color
Green is result of verdoperoxide
Peroxidative enzyme present in cytoplasmic granules of MS
Green color fades on exposure to air within 2-3 hours
MICROSCOPIC PATHOLOGY
Histologic Features
Lymph node
Diffuse or partial effacement of architecture
If partial, paracortical involvement with entrapped residual follicles
Single file pattern of infiltration is common in hilum and capsule
Extranodal sites
Effacement of architecture
Diffuse or single file growth pattern depending on degree of stromal reaction
Destructive bone lesions in patients with underlying MPN
↑ megakaryoblasts, erythroblasts, and eosinophilia
Cytologic Features
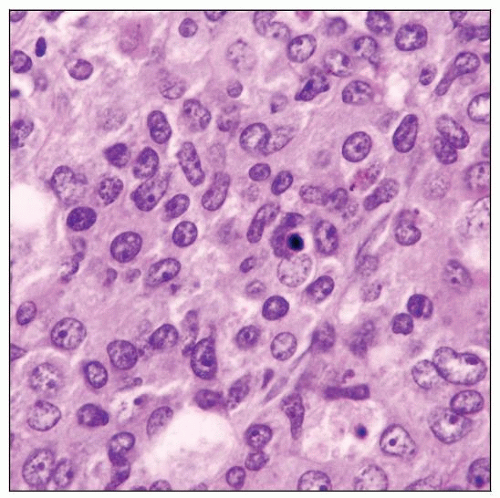
Blasts have thin nuclear membranes, “dusty” chromatin, small nucleoli
High mitotic rate
Most cases of MS are composed of granulocytes with variable differentiation
Eosinophilic myelocytes and metamyelocytes are helpful clues for
Granulocytic differentiation
Diagnosis of MS (in routinely stained tissue sections)
Subset of cases of MS exhibit myelomonocytic or monocytic differentiation
Reniform nuclei are helpful clue for monocytic differentiation
MS cases composed predominantly of megakaryoblasts or erythroblasts are rare
Often a manifestation of MS arising from MPN
Trilineage hematopoiesis is rare in MS
More common in cases arising from MPN
Morphological features of MS can be subdivided according to their degree of differentiation
Different systems have been proposed over the years
No system has prognostic significance
Cytologic features and degree of differentiation often similar in successive relapse specimens
Touch imprints are very helpful
Wright-Giemsa stain allows assessment of morphologic features as seen in bone marrow
Can appreciate dysplasia if present
Unstained, air-dried imprints can be used for cytochemistry
ANCILLARY TESTS
Immunohistochemistry
Sensitivity of various antibodies differs slightly in different studies of MS
CD68/KP-1(+), lysozyme(+), CD43(+) in > 95%
Myeloperoxidase(+): ˜ 90%; CD117(+): ˜ 80%
CD45/LCA(+): 60-70%; CD99(+): 50-60%; CD68/PG-M1(+): ˜ 50%
CD34(+): 40-50%; TdT(+): ˜ 33%; CD56(+): ˜ 15%; CD30(+): < 5%
pax-5(+) and CD19(+) in MS associated with t(8;21) (q22;q22)
CD4(+/-) and CD163(+/-) in cases with monocytic differentiation
Ki-67/MIB1 (proliferation rate) is high (50-95%)
Nucleophosmin (NPM) staining
Cytoplasmic staining correlates with presence of NPM1 gene mutation
Small subset of MS cases can show plasmacytoid monocyte differentiation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree