Organization of a Skeletal Muscle
Organization Within Muscle Fibers
Sarcoplasmic reticulum & Transverse Tubule System
Muscle Spindles & Tendon Organs
Muscle tissue, the fourth basic tissue type with epithelia, connective tissues, and nervous tissue, is composed of cells that optimize the universal cell property of contractility. As in all cells, actin microfilaments and associated proteins generate the forces necessary for the muscle contraction, which drives movement within organ systems, of blood, and of the body as a whole. Essentially all muscle cells are of mesodermal origin and differentiate by a gradual process of cell lengthening with abundant synthesis of the myofibrillar proteins actin and myosin.
Three types of muscle tissue can be distinguished on the basis of morphologic and functional characteristics (Figure 10–1), with the structure of each adapted to its physiologic role.
FIGURE 10–1 The three types of muscle.
 Skeletal muscle contains bundles of very long, multinucleated cells with cross-striations. Their contraction is quick, forceful, and usually under voluntary control.
Skeletal muscle contains bundles of very long, multinucleated cells with cross-striations. Their contraction is quick, forceful, and usually under voluntary control.
 Cardiac muscle also has cross-striations and is composed of elongated, often branched cells bound to one another at structures called intercalated discs that are unique to cardiac muscle. Contraction is involuntary, vigorous, and rhythmic.
Cardiac muscle also has cross-striations and is composed of elongated, often branched cells bound to one another at structures called intercalated discs that are unique to cardiac muscle. Contraction is involuntary, vigorous, and rhythmic.
 Smooth muscle consists of collections of fusiform cells that lack striations and have slow, involuntary contractions.
Smooth muscle consists of collections of fusiform cells that lack striations and have slow, involuntary contractions.
In all types of muscle, contraction is caused by the sliding interaction of thick myosin filaments along thin actin filaments. The forces necessary for sliding are generated by other proteins affecting the weak interactions in the bridges between actin and myosin.
As with neurons, muscle specialists refer to certain muscle cell organelles with special names. The cytoplasm of muscle cells is often called sarcoplasm (Gr. sarkos, flesh + plasma, thing formed), the smooth ER is the sarcoplasmic reticulum, and the muscle cell membrane and its external lamina are the sarcolemma (sarkos + Gr. lemma, husk).
SKELETAL MUSCLE
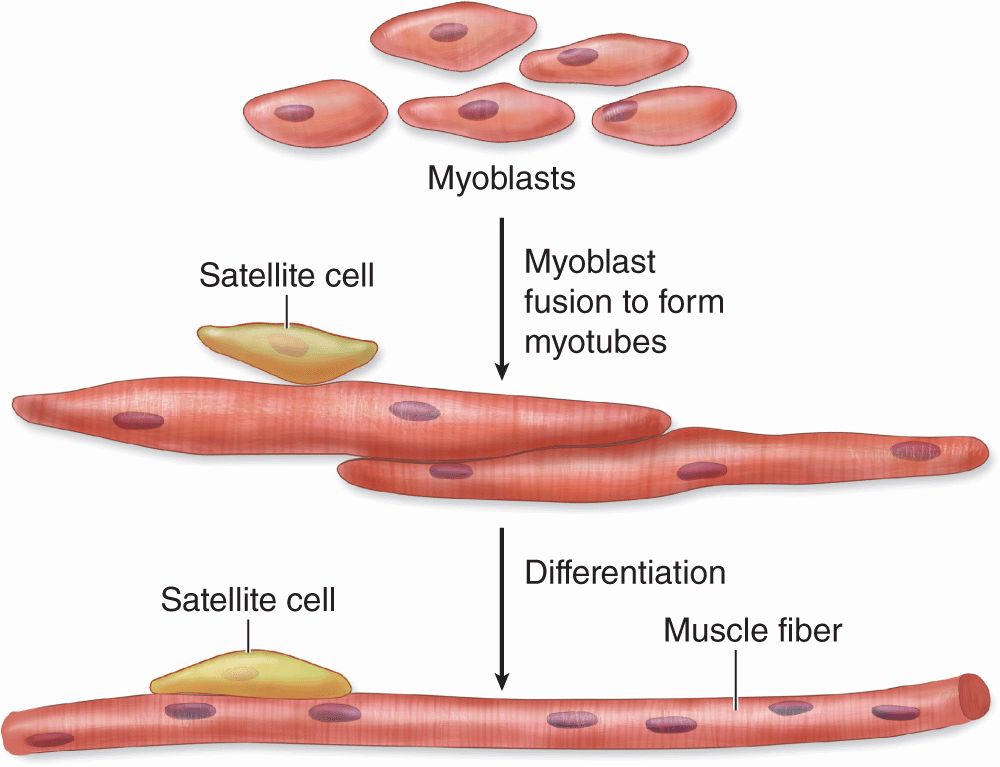
Skeletal (or striated) muscle consists of muscle fibers, which are long, cylindrical multinucleated cells with diameters of 10 to 100 μm. During embryonic muscle development, mesenchymal myoblasts (L. myo, muscle) fuse, forming myotubes with many nuclei. Myotubes then further differentiate to form striated muscle fibers (Figure 10–2). Elongated nuclei are found peripherally just under the sarcolemma, a characteristic nuclear location unique to skeletal muscle fibers/cells. A small population of reserve progenitor cells called muscle satellite cells remains adjacent to most fibers of differentiated skeletal muscle.
FIGURE 10–2 Development of skeletal muscle.
Organization of a Skeletal Muscle
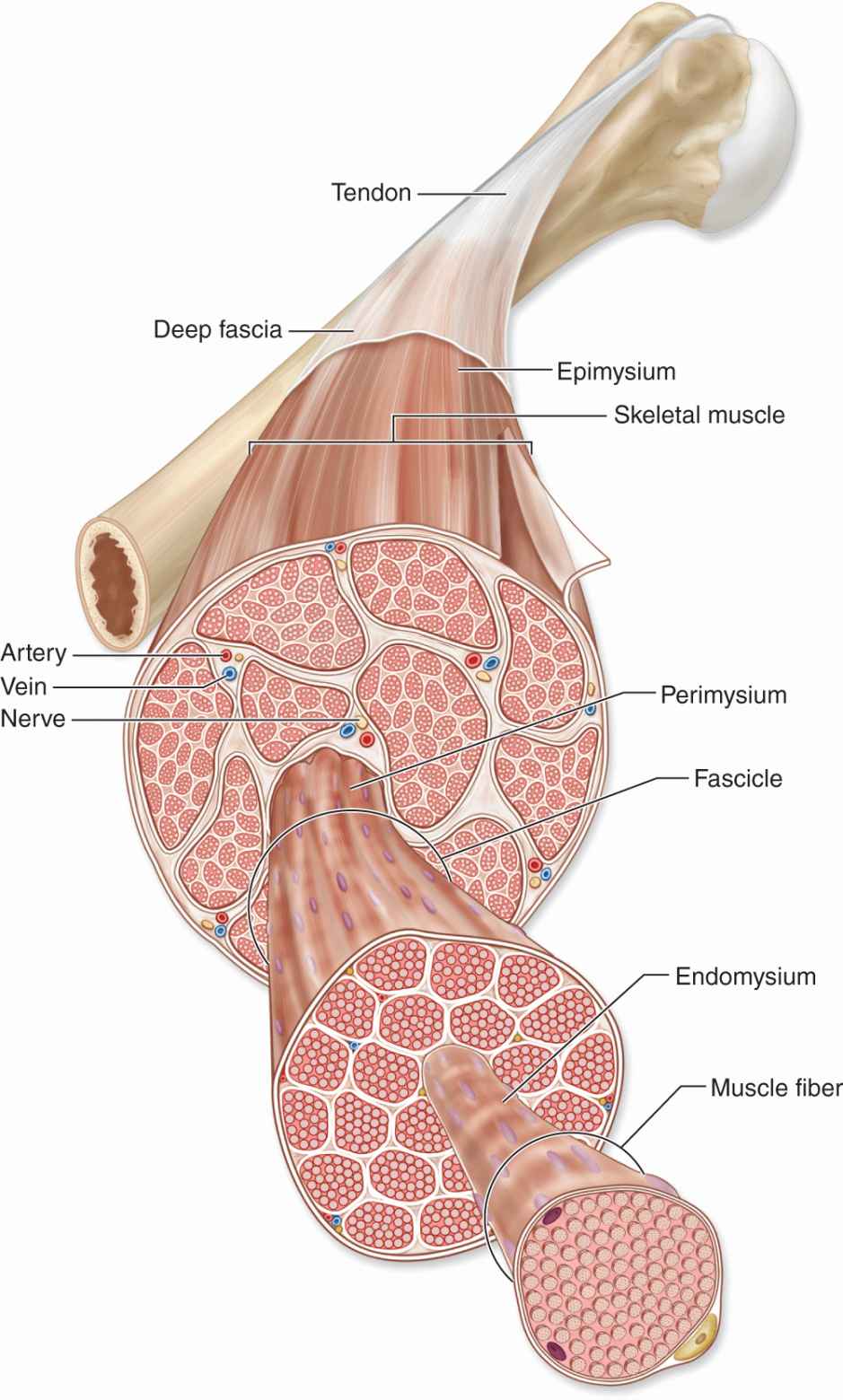
Thin layers of connective tissue surround and organize the contractile fibers in all three types of muscle, and these layers are seen particularly well in skeletal muscle (Figures 10–3 and 10–4). The organization given by these supportive layers resembles that in large peripheral nerves:
FIGURE 10–3 Organization of skeletal muscle.
FIGURE 10–4 Skeletal muscle.
 The epimysium, an external sheath of dense connective tissue, surrounds the entire muscle. Septa of this tissue extend inward, carrying the larger nerves, blood vessels, and lymphatics of the muscle.
The epimysium, an external sheath of dense connective tissue, surrounds the entire muscle. Septa of this tissue extend inward, carrying the larger nerves, blood vessels, and lymphatics of the muscle.
 The perimysium is a thin connective tissue layer that immediately surrounds each bundle of muscle fibers termed a fascicle (Figure 10–3). Each fascicle of muscle fibers makes up a functional unit in which the fibers work together. Nerves, blood vessels, and lymphatics penetrate the perimysium to supply each fascicle.
The perimysium is a thin connective tissue layer that immediately surrounds each bundle of muscle fibers termed a fascicle (Figure 10–3). Each fascicle of muscle fibers makes up a functional unit in which the fibers work together. Nerves, blood vessels, and lymphatics penetrate the perimysium to supply each fascicle.
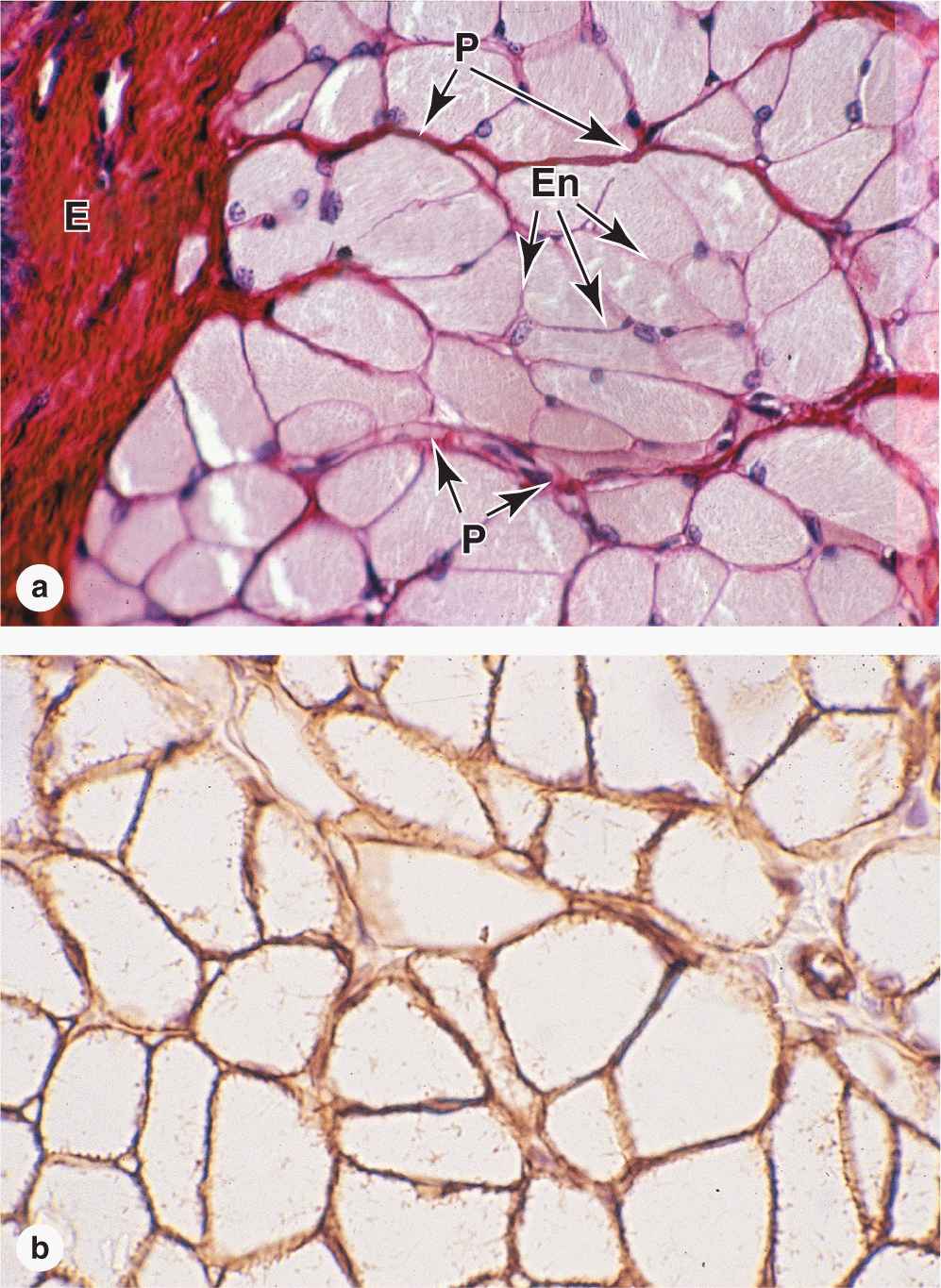
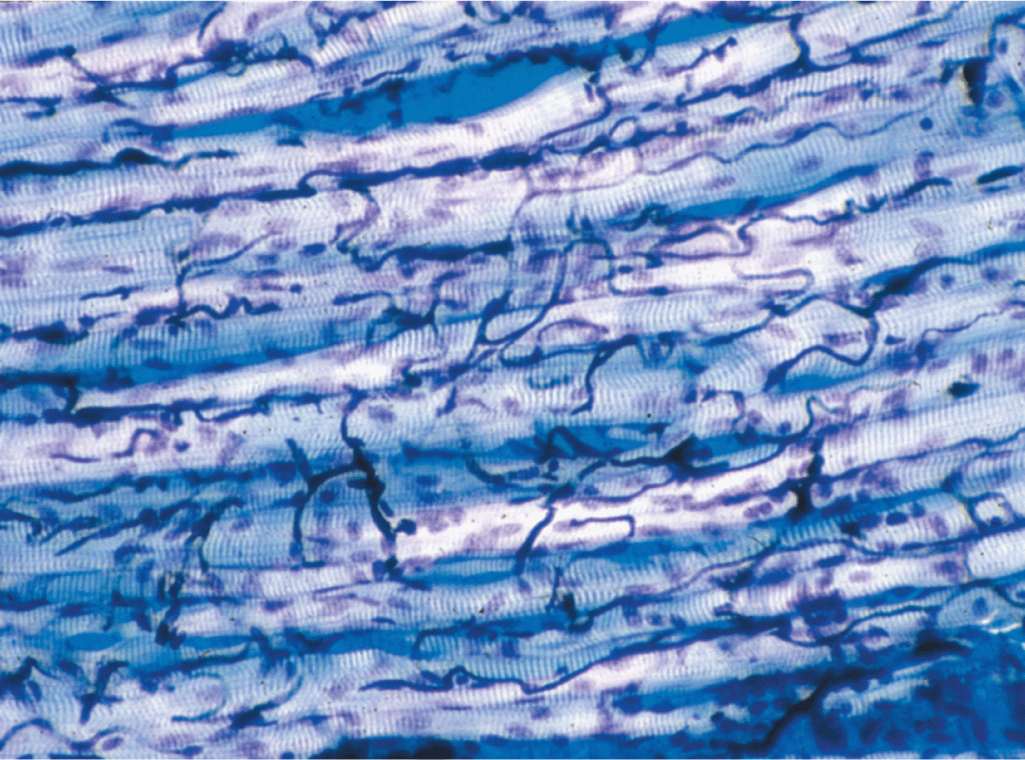
 Within fascicles a very thin, delicate layer of reticular fibers and scattered fibroblasts, the endomysium, surrounds the external lamina of individual muscle fibers. In addition to nerve fibers, capillaries form a rich network in the endomysium bringing O2 to the muscle fibers (Figure 10–5).
Within fascicles a very thin, delicate layer of reticular fibers and scattered fibroblasts, the endomysium, surrounds the external lamina of individual muscle fibers. In addition to nerve fibers, capillaries form a rich network in the endomysium bringing O2 to the muscle fibers (Figure 10–5).
FIGURE 10–5 Capillaries of skeletal muscle.
Collagen in these connective tissue layers of muscle serve to transmit the mechanical forces generated by the contracting muscle cells/fibers; individual muscle fibers seldom extend from one end of a muscle to the other.
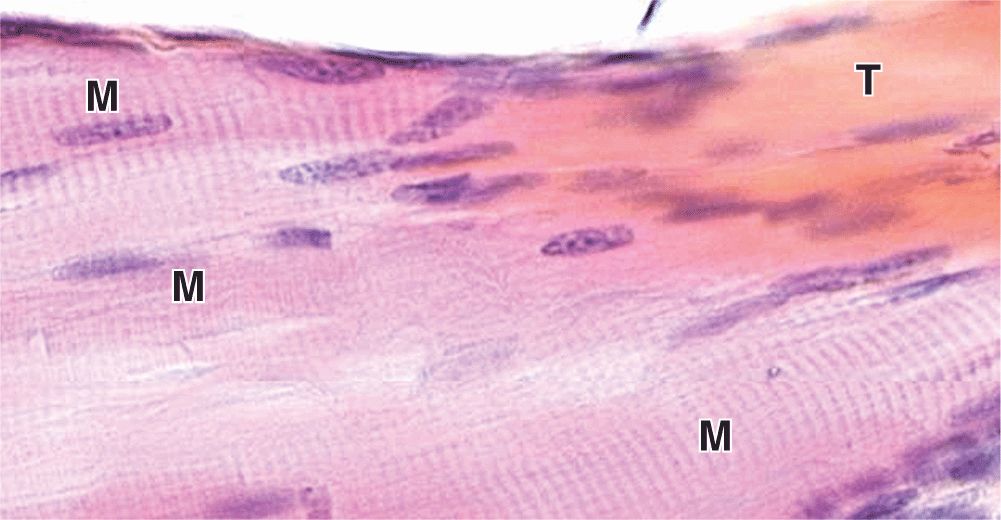
Some skeletal muscles taper at their ends, where the epimysium is continuous with the dense regular connective tissue of a tendon at myotendinous junctions (Figure 10–6). Ultrastructural studies show that in these transitional regions, collagen fibers from the tendon insert themselves among muscle fibers and associate directly with complex infoldings of sarcolemma.
FIGURE 10–6 Myotendinous junction.
Organization Within Muscle Fibers
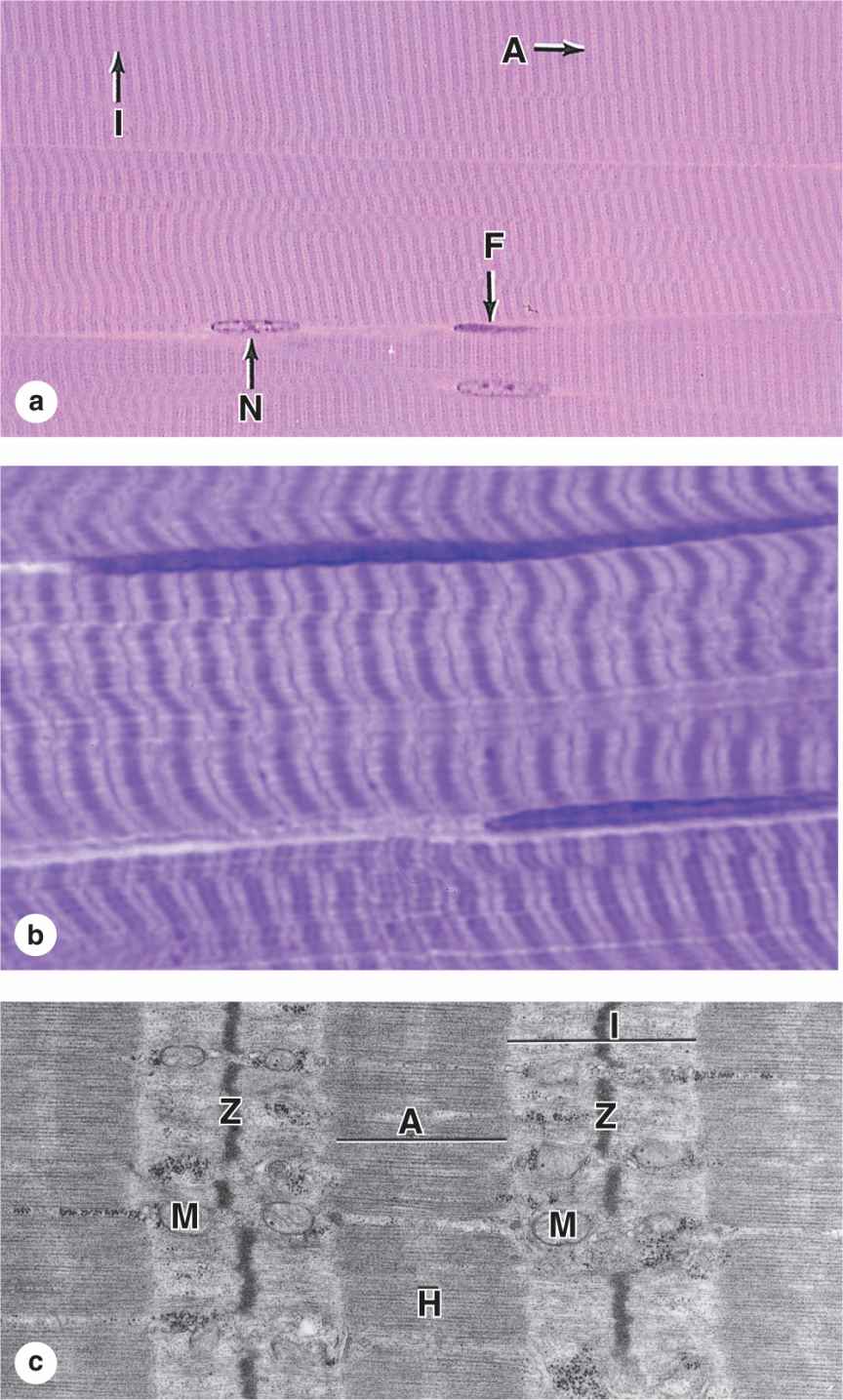
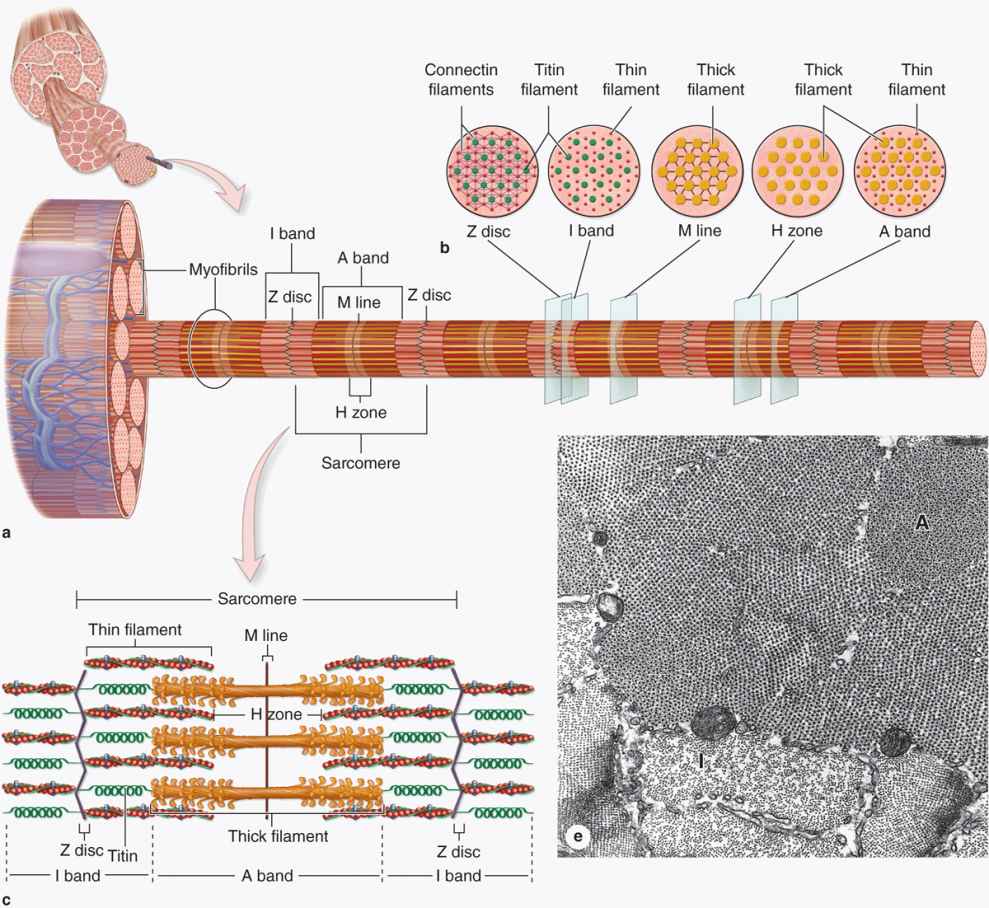
Longitudinally sectioned skeletal muscle fibers show cross-striations of alternating light and dark bands (Figure 10–7). The dark bands are called A bands (anisotropic or birefringent in polarized light microscopy); the light bands are called I bands (isotropic, do not alter polarized light). In the TEM (Figure 10–7c), each I band is seen to be bisected by a dark transverse line, the Z disc (Ger. zwischen, between). The repetitive functional subunit of the contractile apparatus, the sarcomere, extends from Z disc to Z disc (Figure 10–8) and is about 2.5 μm long in resting muscle.
FIGURE 10–7 Striated skeletal muscle in longitudinal section.
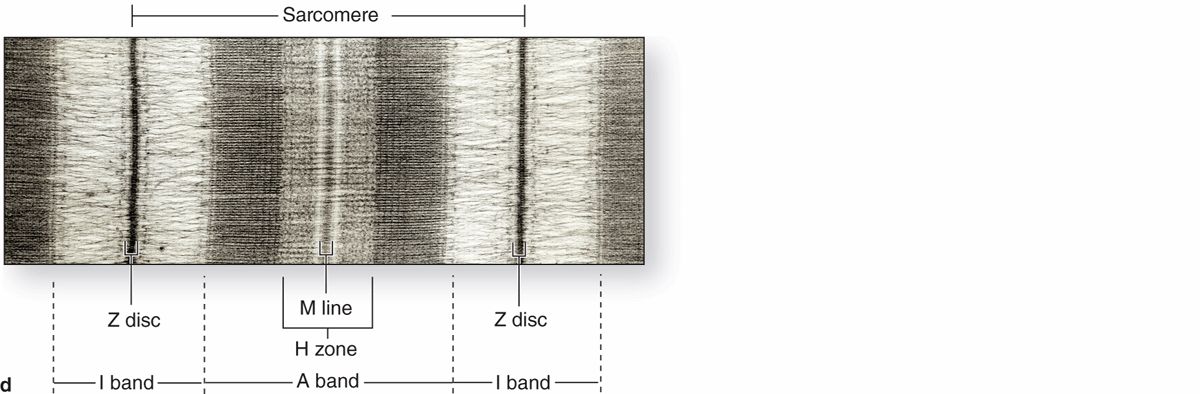
FIGURE 10–8 Structure of a myofibril: A series of sarcomeres.
The sarcoplasm has little RER and contains primarily long cylindrical filament bundles, called myofibrils, running parallel to the long axis of the fiber (Figure 10–8a). Mitochondria and sarcoplasmic reticulum are found between the myofibrils, which have a diameter of 1 to 2 μm. Myofibrils consist of an end-to-end repetitive arrangement of sarcomeres (Figure 10–8a); the lateral registration of sarcomeres in adjacent myofibrils causes the entire muscle fiber to exhibit a characteristic pattern of transverse striations.
The A and I banding pattern in sarcomeres is due mainly to the regular arrangement of thick and thin myofilaments, composed of myosin and F-actin, respectively, organized within each myofibril in a symmetric pattern containing thousands of each filament type.
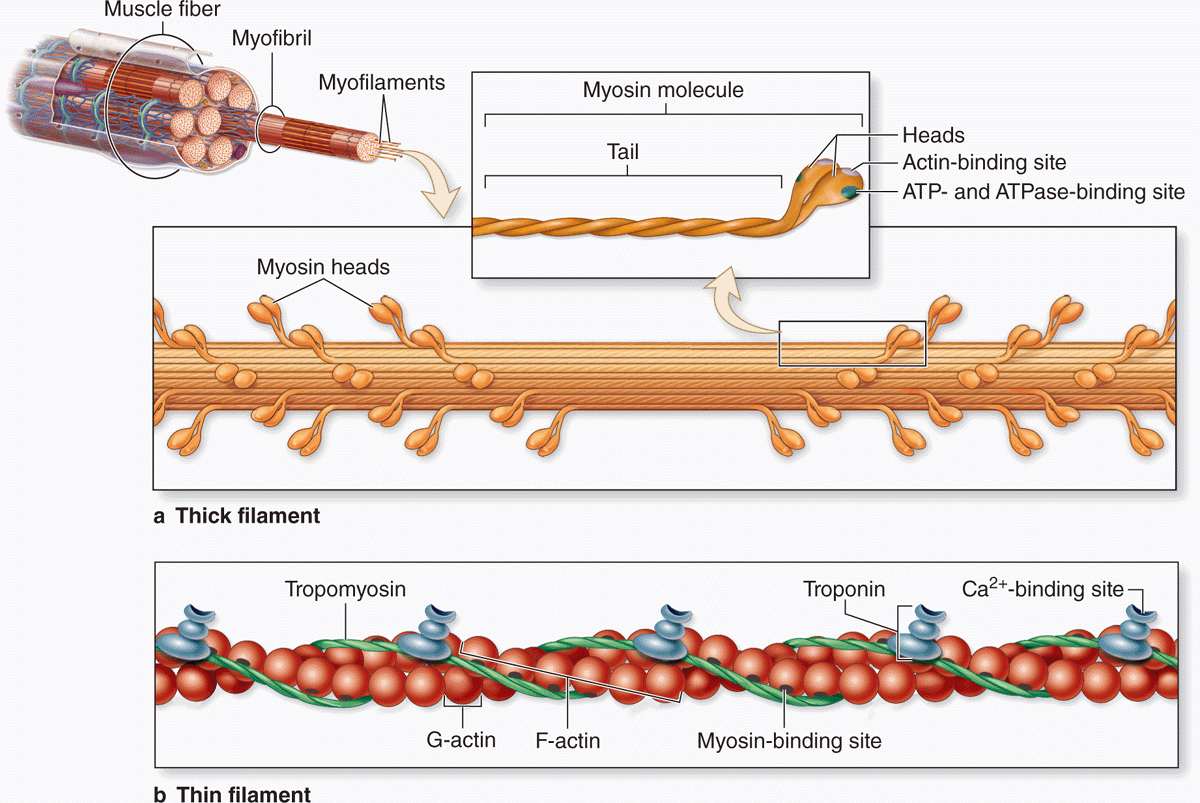
The thick myosin filaments are 1.6 μm long and 15 nm wide; they occupy the A band at the middle region of the sarcomere. Myosin is a large complex (~500 kDa) with two identical heavy chains and two pairs of light chains. Myosin heavy chains are thin, rodlike motor proteins (150 nm long and 2-3 nm thick) twisted together as myosin tails (Figure 10–9). Globular projections containing the four myosin light chains form a head at one end of each heavy chain. The myosin heads bind both actin, forming transient crossbridges between the thick and thin filaments, and ATP, catalyzing energy release (actomyosin ATPase activity). Several hundred myosin molecules are arranged within each thick filament with overlapping rodlike portions and the globular heads directed toward either end (Figure 10–9a).
FIGURE 10–9 Molecules composing thin and thick filaments.
The thin, helical actin filaments are each 1.0 μm long and 8 nm wide and run between the thick filaments. Each G-actin monomer contains a binding site for myosin (Figure 10–9b). Actin filaments are anchored perpendicularly on the Z disc by the actin-binding protein α-actinin and exhibit opposite polarity on each side of this disc (Figure 10–8c). Thin filaments are also tightly associated with two regulatory proteins (Figure 10–9):
 Tropomyosin, a 40-nm-long coil of two polypeptide chains located in the groove between the two twisted actin strands.
Tropomyosin, a 40-nm-long coil of two polypeptide chains located in the groove between the two twisted actin strands.
 Troponin, a complex of three subunits: TnT, which attaches to tropomyosin; TnC, which binds Ca2+; and TnI, which regulates the actin-myosin interaction.
Troponin, a complex of three subunits: TnT, which attaches to tropomyosin; TnC, which binds Ca2+; and TnI, which regulates the actin-myosin interaction.
Troponin complexes attach at specific sites regularly spaced along each tropomyosin molecule.
I bands, each bisected by a Z disc, consist of the portions of the thin filaments that do not overlap the thick filaments (which is why I bands stain more lightly). An important accessory protein in I bands is titin (3700 kDa), the largest protein in the body, with scaffolding and elastic properties, which supports the thick myofilaments and connects them to the Z disc (Figure 10–8c). Another very large accessory protein, nebulin (600-900 kDa), binds each thin myofilament laterally, helps anchor them to a-actinin, and specifies the length of the actin polymers during myogenesis.
The A bands contain both thick filaments and the overlapping portions of thin filaments. Close observation of the A band shows the presence of a lighter zone in its center, the H zone, corresponding to a region with only the rodlike portions of the myosin molecule and no thin filaments (Figure 10–8c). Bisecting the H zone is the M line (Ger. Mitte, middle; Figure 10–8c), containing a myosin-binding protein myomesin that holds the thick filaments in place, and creatine kinase. This enzyme catalyzes transfer of phosphate groups from phosphocreatine, a storage form of high-energy phosphate groups, to ADP, helping to supply ATP for muscle contraction.
Despite the many proteins present in sarcomeres, myosin and actin together represent over half of the total protein in striated muscle. The overlapping arrangement of thin and thick filaments within sarcomeres produces in TEM cross sections hexagonal patterns of structures that were important in determining the functions of the filaments and other proteins in the myofibril (Figures 10–8b and 10-8e).
Sarcoplasmic Reticulum & Transverse Tubule System
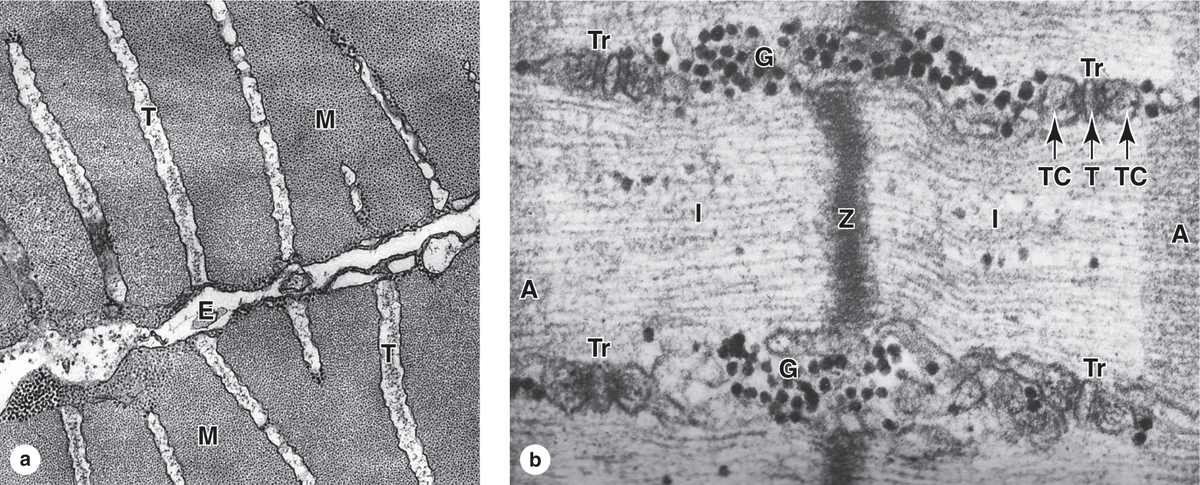
In skeletal muscle fibers the smooth ER, or sarcoplasmic reticulum, is specialized for Ca2+ sequestration. Depolarization of the sarcoplasmic reticulum membrane, which causes release of calcium, is initiated at specialized motor nerve synapses on the sarcolemma. To trigger Ca2+ release from sarcoplasmic reticulum throughout the fiber simultaneously and cause uniform contraction of all myofibrils, the sarcolemma is folded into a system of transverse or T tubules (Figures 10–10a and 10–11. These long fingerlike invaginations of the cell membrane penetrate deeply into the sarcoplasm and encircle every myofibril near the aligned A and I-band boundaries of sarcomeres (Figures 10–10b and 10–11.
FIGURE 10–10 Transverse tubule system.

 MEDICAL APPLICATION
MEDICAL APPLICATION