Macrophages & the Mononuclear Phagocyte System
The different types of connective tissue maintain the form of organs throughout the body. Connective tissues provide a matrix that supports and physically connects other tissues and cells together in organs. The interstitial fluid of connective tissue gives metabolic support to cells as the medium for diffusion of nutrients and waste products.
Unlike the other tissue types (epithelium, muscle, and nerve), which consist mainly of cells, the major constituent of connective tissue is the extracellular matrix (ECM). Extracellular matrices consist of different combinations of protein fibers (such as collagens and elastic fibers) and ground substance. Ground substance is a complex of anionic, hydrophilic proteoglycans, glycosaminoglycans (GAGs), and multiadhesive glycoproteins (laminin, fibronectin, and others). As described briefly in Chapter 4 with the basal lamina, such glycoproteins help stabilize the ECM by binding to other matrix components and to integrins in cell membranes. The hydrated nature of connective tissue ground substance provides the medium for the exchange of nutrients and metabolic wastes between cells and the blood supply.
The variety of connective tissue types in the body reflects differences in composition and amount of the cells, fibers, and ground substance which together are responsible for the remarkable structural, functional, and pathologic diversity of connective tissue.
Connective tissues originate from embryonic mesenchyme, a tissue developing mainly from the middle layer of the embryo, the mesoderm (Figure 5–1). Mesenchyme consists largely of viscous ground substance with few collagen fibers. Mesenchymal cells are undifferentiated and have large nuclei, with prominent nucleoli and fine chromatin. They are often said to be “spindle-shaped,” with their scant cytoplasm extended as two or more thin cytoplasmic processes. Mesodermal cells migrate from their site of origin in the embryo, surrounding and penetrating developing organs. In addition to producing all types of connective tissue proper and the specialized connective tissues bone and cartilage, the embryonic mesenchyme includes stem cells for other tissues such as blood, the vascular endothelium, and muscle. This chapter focuses on connective tissue proper.
FIGURE 5–1 Embryonic mesenchyme.
CELLS OF CONNECTIVE TISSUE
Fibroblasts and certain other cells are typically present in connective tissue proper (Figure 5–2 and Table 5–1). Fibroblasts originate locally from mesenchymal cells and are permanent residents of connective tissue; other cells found here, such as macrophages, plasma cells, and mast cells, originate from hematopoietic stem cells in bone marrow, circulate in the blood, and then move into connective tissue where they function. White blood cells (leukocytes) are transient cells of most connective tissues; they also originate in the bone marrow and move to the connective tissue where they function for a few days, then die by apoptosis.
FIGURE 5–2 Cellular and extracellular components of connective tissue.
TABLE 5–1 Functions of cells in connective tissue proper.
Fibroblasts
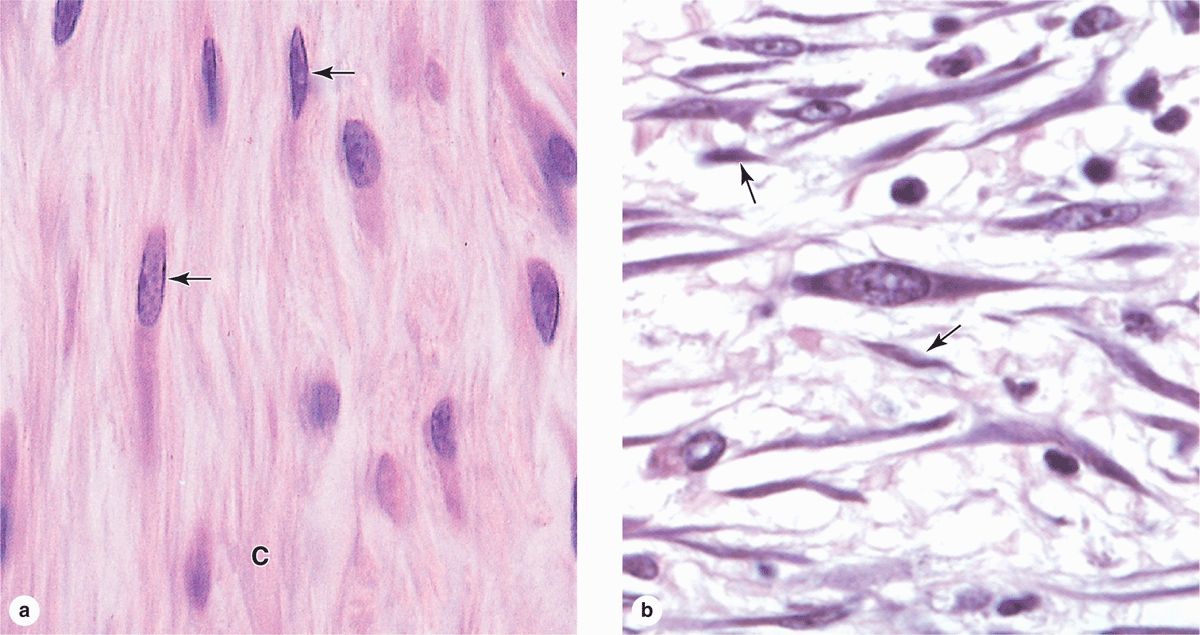
Fibroblasts (Figure 5–3), the most common cells in connective tissue, produce and maintain most of the tissue’s extracellular components. Fibroblasts synthesize and secrete collagen (the most abundant protein of the body) and elastin, which form large fibers, as well as the GAGs, proteoglycans, and multiadhesive glycoproteins that comprise the ground substance. As described later, most of the secreted ECM components undergo further modification outside the cell before assembling as a matrix.
FIGURE 5–3 Fibroblasts.
Two levels of fibroblast activity can be observed histologically (Figure 5–3b). Cells with intense synthetic activity are morphologically distinct from the quiescent fibroblasts that are scattered within the matrix they have already synthesized. Some histologists reserve the term “fibroblast” to denote the active cell and “fibrocyte” to denote the quiescent cell. The active fibroblast has more abundant and irregularly branched cytoplasm. Its nucleus is large, ovoid, euchromatic, and has a prominent nucleolus. The cytoplasm has much rough endoplasmic reticulum (RER) and a well-developed Golgi apparatus. The quiescent cell is smaller than the active fibroblast, is usually spindle-shaped with fewer processes and much less RER, and contains a darker, more heterochromatic nucleus.
Fibroblasts are targets of many families of proteins called growth factors that influence cell growth and differentiation. In adults, connective tissue fibroblasts rarely undergo division. However, stimulated by locally released growth factors, cell cycling and mitotic activity resume when the tissue requires additional fibroblasts, for example, to repair a damaged organ. Fibroblasts involved in wound healing, sometimes called myo-fibroblasts, have a well-developed contractile function and are enriched with a form of actin also found in smooth muscle cells.
Adipocytes
Adipocytes (L. adeps, fat + Gr. kytos, cell), or fat cells, are found in connective tissue of many organs. These large, mesenchymally derived cells are specialized for cytoplasmic storage of lipid as neutral fats, or less commonly for the production of heat. The large deposits of fat in the cells of adipose connective tissue also serve to cushion and insulate the skin and other organs. Adipocytes have major metabolic significance with medical importance and are described and discussed in Chapter 6.
Macrophages & the Mononuclear Phagocyte System
Macrophages are characterized by their well-developed phagocytic ability and specialize in turnover of protein fibers and removal of dead cells, tissue debris, or other particulate material. They have a wide spectrum of morphologic features corresponding to their state of functional activity and to the tissue they inhabit. A typical macrophage measures between 10 and 30 μm in diameter and has an eccentrically located, oval or kidney-shaped nucleus. Macrophages are present in the connective tissue of most organs and are often referred to by pathologists as “histiocytes.”
In the TEM, macrophages are shown to have a characteristic irregular surface with pleats, protrusions, and indentations, a morphologic expression of their active pinocytotic and phagocytic activities (Figure 5–4). They generally have a well-developed Golgi apparatus and many lysosomes.
FIGURE 5–4 Macrophage ultrastructure.
Macrophages derive from bone marrow precursor cells that divide, producing monocytes that circulate in the blood. These cells cross the epithelial wall of venules to penetrate connective tissue, where they differentiate further, mature, and acquire the morphologic features of phagocytic cells. Therefore, monocytes and macrophages are the same cell at different stages of maturation. Macrophages play an important role in the early stages of repair after tissue damage, and under such conditions of inflammation these cells accumulate in connective tissue by local proliferation of macrophages in addition to monocyte recruitment from the blood. Macrophages are distributed throughout the body and are present in most organs. Along with other monocyte-derived cells, they comprise a family of cells called the mononuclear phagocyte system (Table 5–2). The macrophage-like cells have been given different names in different organs, for example Kupffer cells in the liver, microglial cells in the central nervous system, Langerhans cells in the skin, and osteoclasts in bone tissue. However, all are derived from monocytes. All are long-living cells and may survive for months in the tissues. In addition to debris removal, these cells are highly important for the uptake, processing, and presentation of antigens for lymphocyte activation, a function discussed later with the immune system. The transformation from monocytes to macrophages in connective tissue involves increases in cell size, increased protein synthesis, and increases in the number of Golgi complexes and lysosomes.
TABLE 5–2 Distribution and main functions of the cells of the mononuclear phagocyte system.
Mast Cells
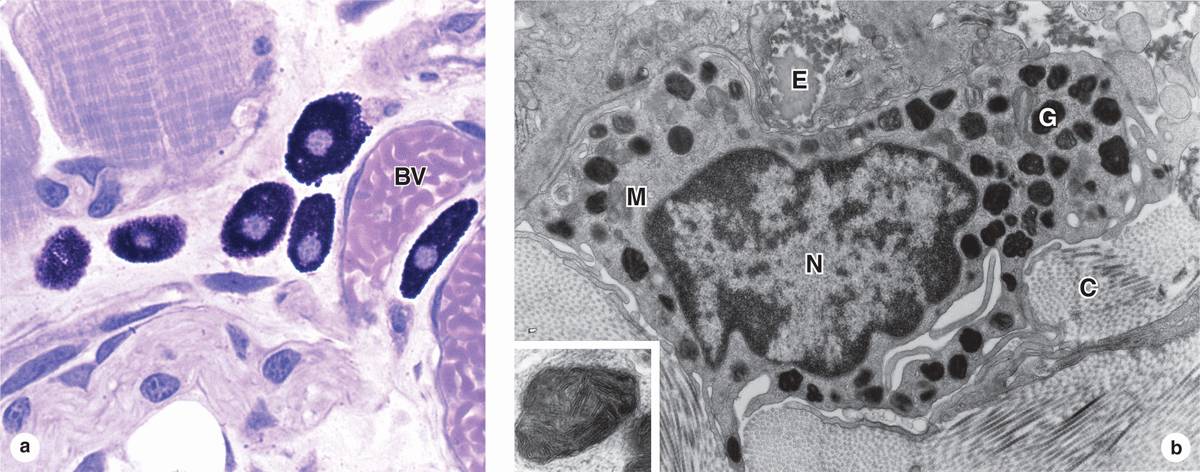
Mast cells are oval or irregularly shaped connective tissue cells, between 7 and 20 μm in diameter, whose cytoplasm is filled with basophilic secretory granules. The nucleus is centrally situated and often obscured by abundant secretory granules (Figure 5–5). These granules are electron-dense and heterogeneous (ranging from 0.3 to 2.0 μm in diameter.) Because of their high content of acidic radicals in their sulfated GAGs, mast cell granules display metachromasia, which means that they can change the color of some basic dyes (eg, toluidine blue) from blue to purple or red. The granules are poorly preserved by common fixatives, so that mast cells are frequently difficult to identify.
FIGURE 5–5 Mast cells.
Mast cells function in the localized release of many bioactive substances with roles in the local inflammatory response, innate immunity, and tissue repair. A partial list of important molecules released from these cells’ secretory granules includes the following:
 Heparin, a sulfated GAG that acts locally as an anticoagulant
Heparin, a sulfated GAG that acts locally as an anticoagulant
 Histamine, which promotes increased vascular permeability and smooth muscle contraction
Histamine, which promotes increased vascular permeability and smooth muscle contraction
 Serine proteases, which activate various mediators of inflammation
Serine proteases, which activate various mediators of inflammation
 Eosinophil and neutrophil chemotactic factors, which attract those leukocytes
Eosinophil and neutrophil chemotactic factors, which attract those leukocytes
 Cytokines, polypeptides directing activities of leukocytes and other cells of the immune system
Cytokines, polypeptides directing activities of leukocytes and other cells of the immune system
 Phospholipid precursors for conversion to prostaglandins, leukotrienes, and other important lipid mediators of the inflammatory response.
Phospholipid precursors for conversion to prostaglandins, leukotrienes, and other important lipid mediators of the inflammatory response.
Occurring in connective tissue of many organs, mast cells are especially numerous near small blood vessels in skin and mesenteries (perivascular mast cells) and in the tissue that lines digestive and respiratory tracts (mucosal mast cells); the granule content of the two populations differs somewhat. These major locations suggest that mast cells place themselves strategically to function as sentinels detecting invasion by microorganisms.
Mast cells originate from progenitor cells in the bone marrow. The progenitor cells circulate in the blood, cross the wall of venules and capillaries, and penetrate connective tissues, where they differentiate. Although they are in many respects similar to basophilic leukocytes, they appear to have a different lineage at least in humans.
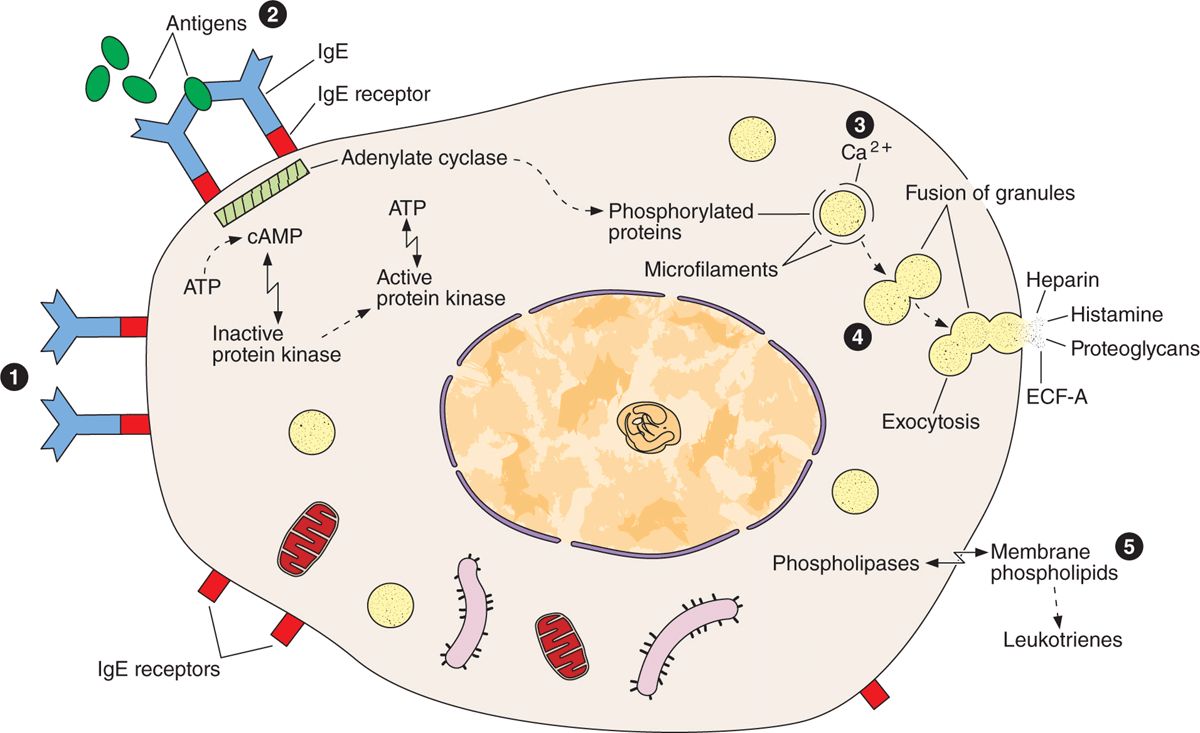
Release of certain chemical mediators stored in mast cells also promotes the allergic reactions, also known as immediate hypersensitivity reactions because they occur within a few minutes after the appearance of an antigen in an individual previously sensitized to the same or a very similar antigen. There are many examples of immediate hypersensitivity reaction; a dramatic one is anaphylactic shock, a potentially fatal condition. The process of anaphylaxis consists of the following sequential events. The first exposure to an antigen (allergen), such as bee venom, results in production of the immunoglobulin E (IgE) class of immunoglobulins (antibodies) by plasma cells. IgE is avidly bound to the surface of mast cells. A second exposure to the antigen results in binding of the antigen to IgE on the mast cells. This event triggers release of the mast cell granules, liberating histamine, leukotrienes, chemokines, and heparin (Figure 5–6). Degranulation of mast cells also occurs as a result of the action of the complement molecules that participate in the immunologic reactions described in Chapter 14.
FIGURE 5–6 Mast cell secretion.
Plasma Cells
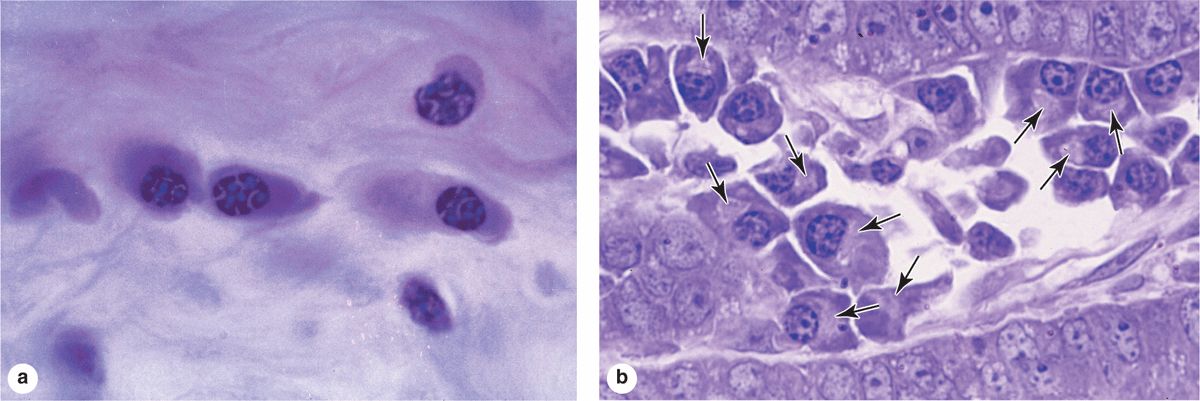
Plasma cells are B-lymphocyte-derived, antibody-producing cells. These large, ovoid cells have basophilic cytoplasm due to their richness in RER (Figure 5–7). Next to the nucleus, the Golgi apparatus and centrioles occupy a region that may appear pale in routine histologic preparations (Figure 5–7b).
FIGURE 5–7 Plasma cells
The nucleus of the plasma cell is generally spherical but eccentrically placed. Many of these nuclei contain compact, peripheral regions of heterochromatin alternating with lighter areas of euchromatin, a configuration that can give the nucleus of a plasma cell the appearance of a clock face. There are at least a few plasma cells in most connective tissues. Their average lifespan is only 10-20 days.
Leukocytes
Besides macrophages and plasma cells, connective tissue normally contains other leukocytes derived from cells circulating in the blood. Leukocytes, or white blood cells, make up a population of wandering cells in connective tissue. They leave blood by migrating between the endothelial cells lining venules to enter connective tissue by a process called diapedesis. This process increases greatly during inflammation, which is a vascular and cellular defensive response to injury or foreign substances, including pathogenic bacteria or irritating chemical substances.
Classically, the major signs of inflamed tissues include “redness and swelling with heat and pain” (rubor et tumor cum calore et dolore). Inflammation begins with the local release of chemical mediators from various cells, the ECM, and blood plasma proteins. These substances act on the local microvasculature, mast cells, macrophages, and other cells to induce events characteristic of inflammation, for example increased blood flow and vascular permeability, diapedesis and migration of leukocytes, and activation of macrophages for phagocytosis.
Most leukocytes function for a few hours or days in connective tissue and then die. However, as discussed with the immune system, some lymphocytes and phagocytic antigen-presenting cells normally leave the interstitial fluid of connective tissue, enter blood or lymph, and move to selected lymphoid organs.
FIBERS
The fibrous components of connective tissue are elongated structures formed from proteins that polymerize after secretion from fibroblasts (Figure 5–2). The three main types of fibers include collagen, reticular, and elastic fibers. Collagen and reticular fibers are both formed by proteins of the collagen family, and elastic fibers are composed mainly of the protein elastin. These fibers are distributed unequally among the different types of connective tissue, with the predominant fiber type usually responsible for conferring specific tissue properties.
Collagen
The collagens constitute a family of proteins selected during evolution for their ability to form a variety of extracellular structures. The various fibers, sheets, and networks made of collagens are all extremely strong and resistant to normal shearing and tearing forces. Collagen is a key element of all connective tissues, as well as epithelial basement membranes and the external laminae of muscle and nerve cells.
Collagen is the most abundant protein in the human body, representing 30% of its dry weight. A major product of fibroblasts, collagens are secreted by several other cell types and are distinguishable by their molecular compositions, morphologic characteristics, distribution, functions, and pathologies. A family of 28 collagens exists in vertebrates, the most important of which are listed in Table 5–3. They can be grouped into the following categories according to the structures formed by their interacting subunits:
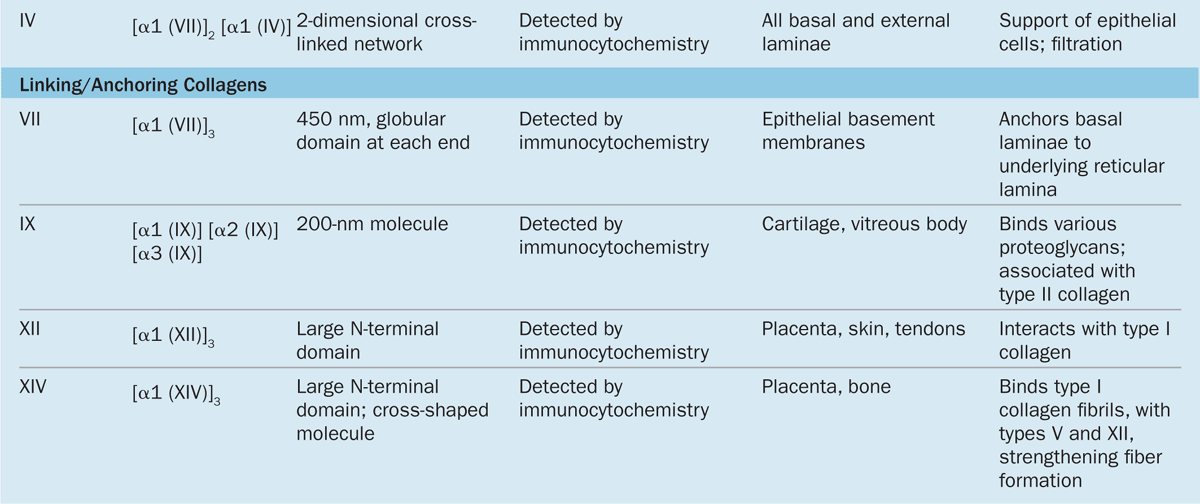
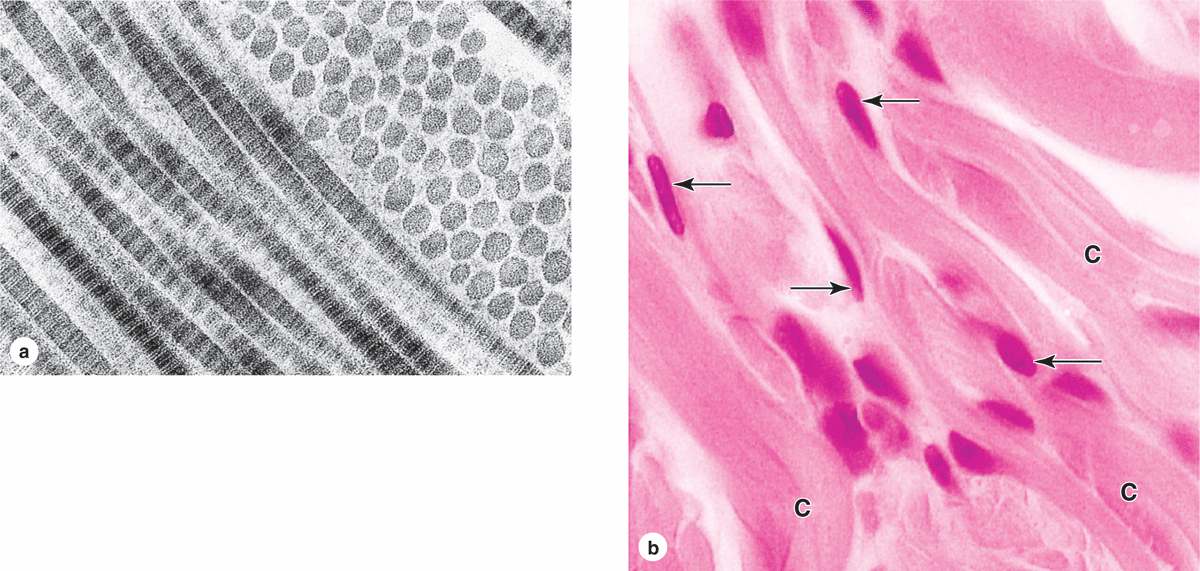
 Fibrillar collagens, notably collagen types I, II, and III, have subunits that aggregate to form large fibrils clearly visible in the electron or light microscope (Figure 5–8). Collagen type I, the most abundant and widely distributed collagen, forms large, eosinophilic bundles usually called collagen fibers. These often densely fill the connective tissue, forming structures such as tendons, organ capsules, and dermis.
Fibrillar collagens, notably collagen types I, II, and III, have subunits that aggregate to form large fibrils clearly visible in the electron or light microscope (Figure 5–8). Collagen type I, the most abundant and widely distributed collagen, forms large, eosinophilic bundles usually called collagen fibers. These often densely fill the connective tissue, forming structures such as tendons, organ capsules, and dermis.
FIGURE 5–8 Type I collagen.
 MEDICAL APPLICATION
MEDICAL APPLICATION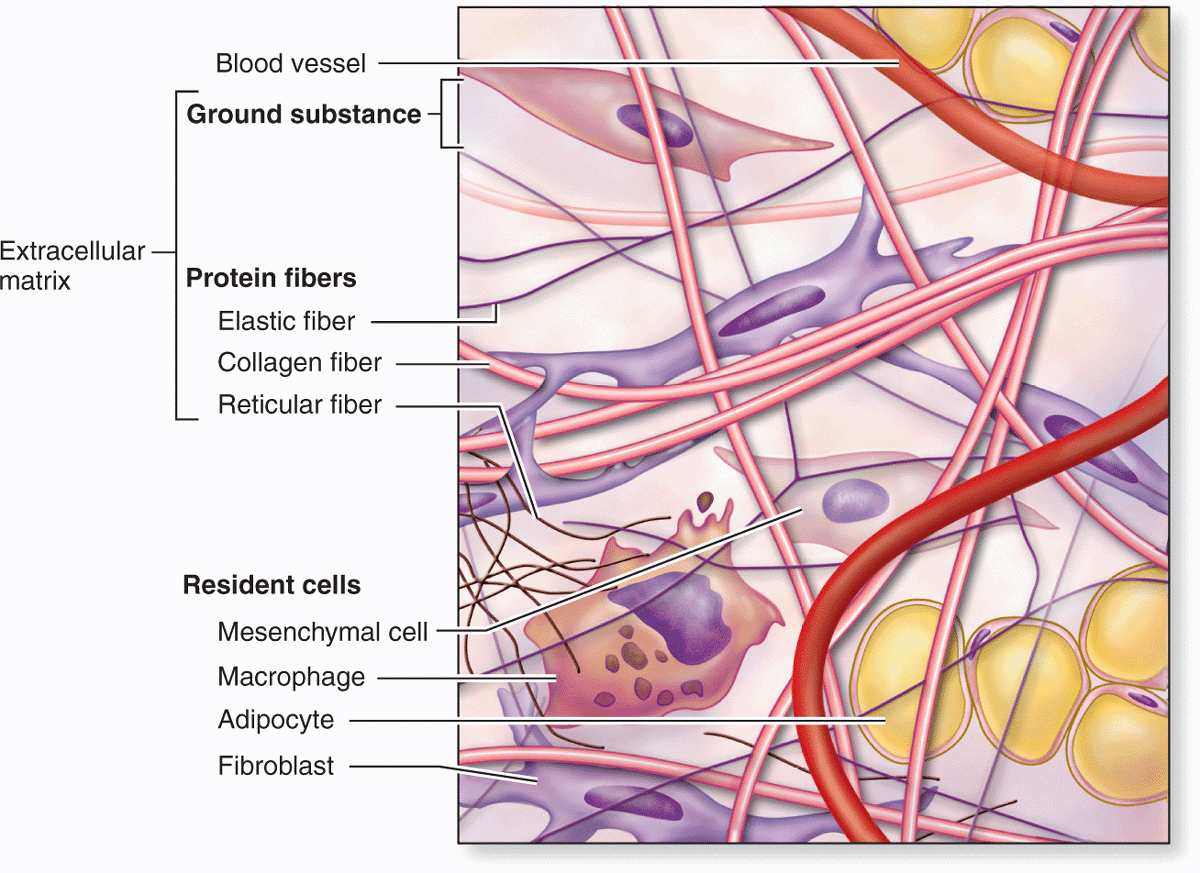
 MEDICAL APPLICATION
MEDICAL APPLICATION MEDICAL APPLICATION
MEDICAL APPLICATION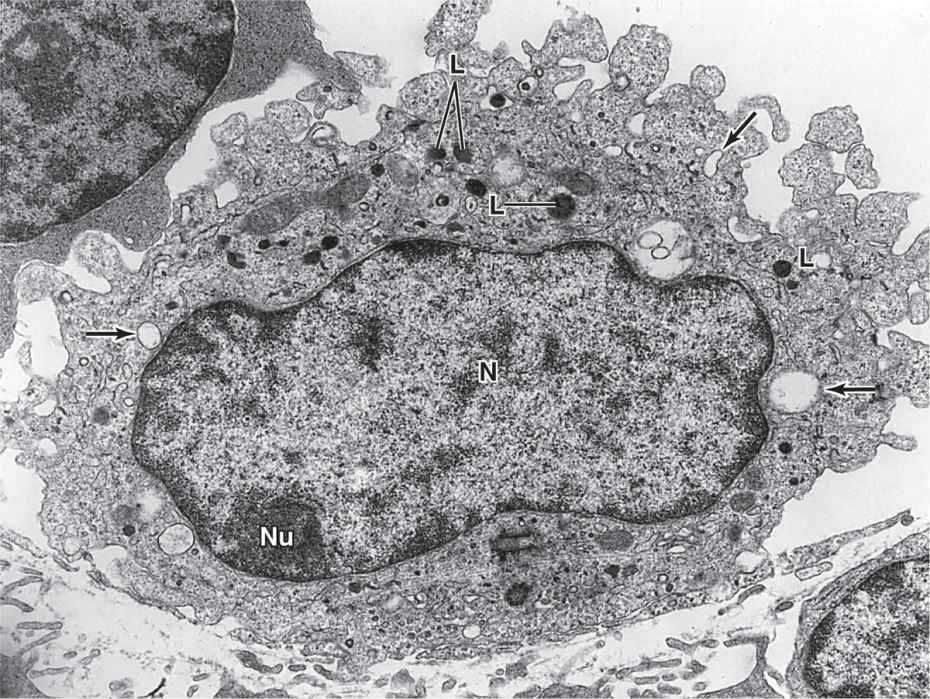
 MEDICAL APPLICATION
MEDICAL APPLICATION MEDICAL APPLICATION
MEDICAL APPLICATION