Fig 10.1
Human tear film comprise of the lipid layer followed by the aqueous layer resting on the glycocalyx matrix of mucin layer on the corneal epithelium
Mucins are large, extracellular glycoproteins with molecular weights ranging from 0.5 to 20 MDa and belong to the family having O-linked carbohydrates. They are classified as transmembrane or secretory mucins. Secreted mucins can be further subclassified as gel forming or soluble, based on their ability to form polymers. They are highly glycosylated, containing 80 % of carbohydrates, primarily N-acetylgalactosamine, N-acetylglucosamine, fucose, galactose, and sialic acid (N-acetylneuraminic acid), and traces of mannose and sulfate. Both secreted and membrane bound forms of mucins share many common features (Bansil and Turner 2006). Mucins that have been detected in the eye are MUC1, MUC2, MUC4, MUC5AC, MUC7, MUC13, MUC15, MUC16, and MUC17 (Johnson and Murphy 2004). Transmembrane mucins contain hydrophobic, membrane-spanning domains in their carboxyl-terminal region, which anchor them to the apical surface of conjunctival and corneal epithelial cells, facilitating formation of the ocular surface glycocalyx (Johnson and Murphy 2004; Tiffany 2008). MUC5AC, the major gel-forming mucin of tears, is secreted by conjunctival goblet cells. This complex macromolecule gives interface for holding moisture on the biological membrane due to various non-covalent interactions.
The primary function of tear film includes providing nutrition to the epithelium of the cornea, lubricating ocular surface, and removal of debris from the precorneal area along with the factors for antimicrobial and immune functions. Tear film stability is achieved by the coordinated functions of lipid monolayer with its critical composition and the contents of aqueous interface. Tear instability causes dry eye due to various factors; any disturbance in the composition of the outer lipid layer can lead to the loss of primary defense of tear evaporation (Gipson and Argüeso 2003). Deficiency in mucin and aqueous volume (tear turnover rate) are the other factors responsible for destabilization of tear film integrity leading toward dryness.
10.3 Drugs used in Dry eye condition
10.3.1 Mucoadhesive Polymers for Ocular Surface
Conjunctival goblet cells in the eye secrete mucin which coats the epithelial cells to convert the hydrophobic epithelium to hold water against gravity which in turn maintains tear integrity. Goblet cells intercalated within the stratified epithelium of the conjunctiva secrete the large gel-forming mucin MUC5AC, and lacrimal gland epithelia secrete the small soluble mucin MUC7. Apical cells of the stratified epithelium of both corneal and conjunctival epithelium express at least three membrane-associated mucins (MUCs 1, 4, and 16), which extend from their apical surface to form the thick glycocalyx at the epithelium-tear film interface (Johnson and Murphy 2004). This interface is responsible for the conversion of the property of hydrophobic corneal epithelium into hydrophilic layer to hold tear of 7 μm thickness upon the cornea against gravity. In the inadequacy of the function of mucin, mechanical forces during blinking can result in inflammation of the ocular surface (Itakura et al. 2013). Therefore, efforts were made to constitute artificial tear solutions with mucoadhesive polymers, extracellular cations with a suitable pH and osmolarity for the management of dry eye syndrome.
Mucoadhesion is the property of some of the natural or synthetic macromolecules which can adhere to a biological surface and retain their presence for a prolonged duration. Polyvinylpyrrolidone (PVP), polyvinyl alcohol (PVA), methyl cellulose, hydroxypropyl methyl cellulose (HPMC), carboxymethyl cellulose, hyaluronic acid, polyethylene glycols, etc., are some of the polymers regularly used in most of the topical tear substitutes. However, mucoadhesive polymers such as thiolated poly(acrylic acid), poloxamer, celluloseacetophthalate, methyl cellulose, hydroxy ethyl cellulose, poly(amidoamine) dendrimers, poly(dimethyl siloxane), and poly(vinyl pyrrolidone) are also used for increasing the precorneal drug residence time in ocular drug delivery approaches (Wagh et al. 2008).
These bioadhesive polymers used in topical tear substitutes are known to have numerous hydroxyl or carboxyl functional groups having the capability of making hydrogen bond with water molecules in the precorneal area. In ophthalmic products, bioadhesive pre-swollen polymers in water are used in a concentration to exhibit low viscosity. In the swollen state the interdistance between their chains leading to polymer flexibility causes uniform spreading upon the cornea and conjunctiva. Occurrence of polymer-mucin force of interaction has been reported when mucoadhesive polymers interact with corneal epithelium. Using in vitro and in vivo visometric data, mucoadhesive polymer interaction has been defined as polymer-cumin force of interaction in precorneal area. Depleting precorneal mucin by treatment with N-acetylcysteine reduced this interaction significantly (Saettone et al. 1994).
10.3.1.1 Tear Substitutes
Cellulose derivatives such as HPMC, methyl cellulose, and hyaluronic acid all share a common finding of having an –OH group to make hydrogen bonding and hydrophobic methyl groups (Fig. 10.2). The amphiphilic nature of these polymers would be having required hydrophobicity to bind toward hydrophobic epithelium and get entangled in the glycocalyx matrix, thereby giving a required polyhydric hydroxyl groups, creating hydrogen bonding with water molecules. This function makes them as a substitute for mucin in artificial tears. Solutions of hydroxypropyl methylcellulose (HPMC) and polyvinyl alcohol (PVA) are widely used as artificial tears. However, their usefulness is limited by the short duration of their effect. Dilute sodium hyaluronate solutions exhibit non-Newtonian rheology with high viscosities at low shear rates, which would be expected to enhance their ocular surface residence time (Snibson et al. 1992). Although several combinations are available worldwide either with single or more than one polymer, so far they are not studied for their compositional variation to make it more suitable for its use in tear substitute.
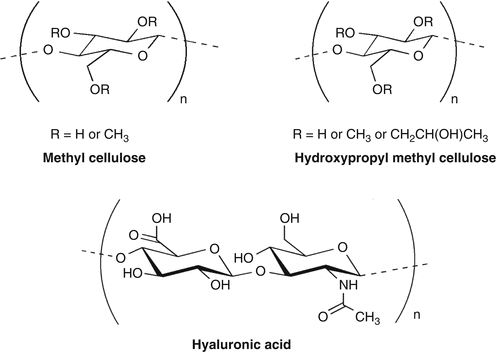

Fig 10.2
Tear Substitutes-Cellulose derivatives used in dry eye disease; HPMC, methyl cellulose, and hyaluronic acid
Throughout the world several compositions of artificial tear are being used and studied in different settings for dry eye. A meta-analysis reviewed 51 of such studies and reported that nearly all formulations of artificial tears provided significant benefit to patients with dysfunctional tear syndrome, but some proved superior to others (Moshirfar et al. 2014). This shows that artificial tear holds a prime position in the management of dry eye syndrome.
Although artificial tear solutions are mostly preferred for dry eye syndrome, at times ophthalmic ointments are used for prolonged management during ocular surgery or enabling nighttime applications. Changing refractive index between the tears and ointment causes blurring of vision and has been considered as one of the major disadvantages of ointments.
10.3.1.2 Other Ophthalmic Uses of Mucoadhesive Polymers
Mucoadhesive polymers are extensively used in ophthalmology during surgical procedures. Sodium hyaluronate (1 %) is very commonly used as one of the ophthalmic viscoelastic substance during capsulorhexis and intraocular lens (IOL) implantation in phacoemulsification surgery. Sodium hyaluronate, chondroitin, and hydroxypropyl methylcellulose are placed in the anterior chamber to prevent corneal endothelial damage during phacoemulsification and are removed after the completion of the procedure (Hutz et al. 1996). Intracameral injection of 2 % HPMC during trabeculectomy has been reported to maintain anterior chamber depth and reduces incidence of complications related to shallow anterior chamber depth following trabeculectomy (Agarwal et al. 2005).
Extensively, mucoadhesive polymers were studied for their activity to enhance the contact time of drugs on the precorneal area (Bucolo et al. 2011); they are also reported to reduce the ocular irritation of drugs when applied topically.
The conditioning properties of the multipurpose contact lens solution with polymers such as HPMC showed improved wetting of lenses and enhanced lens wearing comfort. Moreover, it has also been suggested that binding of HPMC to the lens surface and subsequent time-release is the probable mechanism for these benefits (Simmons et al. 2001).
10.3.2 Secretagogue Agents (Tear Stimulants and Mucin Inducers)
10.3.2.1 Tear stimulants
Lacrimal glands having M3 muscarinic receptors are the prime targets for increasing the tear secretion rate. Pilocarpine and cevimeline are the muscarinic agents approved for the treatment of symptoms of xerostomia in Sjögren’s syndrome.
Pilocarpine
Pilocarpine is a well-known muscarinic agonist used for decades for inducing lacrimation. It is an alkaloid isolated from the plant Pilocarpus jaborandi. Being a muscarinic agonist on all muscarinic receptors, its systemic side effects are bothersome. Administration of 5-mg pilocarpine tablets 4 times daily (20 mg/day) was well tolerated and produced significant improvement in symptoms of dry mouth and dry eyes and other xeroses in patients (Vivino et al. 1999).
Cevimeline
Cevimeline is a synthetic compound, chemically ((±)-cis-2-methylspiro [1,3- oxathiolane-5, 3′-quinuclidine]), available as monohydrochloride reported to be an agonist on M1 and M3 receptors. Cevimeline at a dosage of 30 mg 3 times daily resulted in substantive improvement by increasing the rate of saliva and tear flow in patients with Sjögren’s syndrome, as well as improving subjective symptoms of dry mouth, dry eyes, and overall dryness (Petrone et al. 2002). Frequently reported adverse events included headache, increased sweating, abdominal pain, and nausea. Increasing the dose to 60 mg TID increased higher gastrointestinal side effects. However, at the dose of 20 mg three times daily, it has showed significant improvements in the subjective symptoms, tear dynamics, condition of the corneoconjunctival epithelium, and global improvement rating with lesser side effects (Ono et al. 2004)
10.3.2.2 Mucin inducers
Conjunctival epithelial and goblet cell P2Y2 nucleotide receptors regulate ion transport and glycoprotein release onto the ocular surface to promote tear and mucin secretion via elevated intracellular Ca2+ concentrations. Diquafosol tetrasodium (INS-365), a second-generation uridine nucleotide analog reported to act through P2Y2 receptor, induces the secretion of aqueous tear components from conjunctival epithelial cells and secretion of mucin from conjunctival goblet cells, thereby improving corneal epithelial integrity and stabilizing the tear film (Terakado et al. 2014). Diquafosol ophthalmic solution (3 %) is currently approved in Japan and South Korea for the treatment of dry eye.
In patients with dry eye, topical therapy with diquafosol significantly improved fluorescein and rose bengal staining scores as compared with placebo. Topical solution of 3 %, one drop, four times a day, was reported as noninferior to sodium hyaluronate ophthalmic solution 0.1 % (Keating 2015). Based on the published randomized clinical trial data on the use of diquafosol (3 % topical) in the management of dry eye, it has been suggested that topical therapy consistently improved tear film volume and stability (Lau et al. 2014). Whereas it was not accompanied with major improvement in symptoms related to dry eye disease. This could be due to the complex nature of dry eye disease having multiple etiologies. In most of the cases, the treatment period required was also long (6 months) to get subjective and objective improvement in aqueous-deficient dry eye, whereas topical diquafosol therapy was effective for patients with obstructive meibomian gland dysfunction (Arita et al. 2013). Diquafosol ophthalmic solution 3 % was generally well tolerated in patients with dry eye, with eye irritation the most commonly reported adverse event (Keating 2015).
10.3.2.3 Rebamipide
Rebamipide is initially developed as a gastroprotective compound capable of inducing mucin secretion through prostaglandin generation in the gastric mucosa. This compound has been selected from over 500 amino acid analogs of 2(1H)-quinolinone tested for gastroprotective action and for efficacy to heal experimental gastric ulcers (Arakawa et al. 1998) and nonsteroidal anti-inflammatory drug-induced gastric ulcers. MUC5AC has been identified as a major secretory mucin of conjunctival goblet cells and precorneal tear film. It has been found to decrease significantly in patients suffering from dry eye (Zhao et al. 2001). In vitro studies showed that rebamipide stimulated proliferation of conjunctival goblet cells in primary culture (Rios et al. 2006). Oral administration of rebamipide represents a new therapeutic modality in the treatment of Sjogren syndrome (Kohashi et al. 2008). It has been reported to stimulate EGF receptor (EGFR) and p44/p42 mitogen-activated protein kinase (MAPK) to cause mucin secretion from conjunctival goblet cells (Ríos et al. 2008). Rebamipide promoted glycol conjugate, which has a property as a mucin-like glycoprotein, in human corneal epithelial cells. The increased production was mediated by MUC1 and MUC4 gene expression (Takeji et al. 2012).
Being a suspension, it has been reported to affect optical quality by ocular higher-order aberrations and forward light scatter upon its use in patients (Koh et al. 2013)
Clinically, rebamipide ophthalmic suspensions were tested and reported to be effective in restoring tear stability in patients with dry eye. Topical rebamipide has also been shown to be effective in treating other ocular surface disorders such as lagophthalmos, lid wiper epitheliopathy, and persistent corneal erosion Kashima et al. (2015). Oral rebamipide administration for the period of 3 months showed significant levels of rebamipide in the tear film, indicating potential value in management of mucin-deficient tear film dysfunction (Tandon et al. 2012).
10.3.3 Anti-inflammatories and Immunosuppressants
10.3.3.1 Topical Cyclosporine
Cyclosporine-A (CsA) is a lipophilic cyclic undecapeptide, produced by the fungus Beauveria nivea. CsA suppresses humoral immunity and T-cell-dependent immune mechanisms which are responsible for transplant rejection and forms autoimmunity. Systemically administered CsA is metabolized by CYP3A4 and showed severe systemic toxicity such as renal dysfunction, tremor, hirsutism, hypertension, hyperlipidemia, and gum hyperplasia.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


