Mucinous Carcinoma
ADRIANA D. CORBEN
EDI BROGI
Mucinous carcinoma is characterized by abundant production of extracellular mucin with admixed clusters of tumor cells. Other terms used to identify this tumor include gelatinous, colloid, mucous, and mucoid carcinoma.
The specific histologic appearance of mucinous carcinoma has been appreciated for more than 150 years.1,2,3 Some of the earliest descriptions commented on the slow growth rate and favorable prognosis of these tumors. The importance of distinguishing between pure mucinous tumors and those with a nonmucinous component was emphasized by Geschickter4 in 1938. He noted that prognosis varied “with the amount of mucoid substance found” in the tumor. Various criteria have been used to distinguish mucinous carcinoma from infiltrating duct carcinoma with mucinous differentiation. Pure mucinous carcinomas have been described as tumors that have no nonmucinous infiltrating duct carcinoma,5 tumors which were “virtually pure,”6 tumors with at least 50% growing in a mucinous pattern,7 tumors in which extracellular mucin constituted at least 33% of the lesion,8 and tumors with at least 90% mucinous component.9 One author indicated that “these proportions were arbitrarily selected.”8 At present, the designation of pure mucinous carcinoma is best applied to tumors with at least a 90% mucinous component. The term “mixed mucinous carcinoma” should be used for tumors in which the mucinous component represents between 50% and 90% of the lesion. Invasive duct carcinomas with less than 50% mucinous component are best referred to as having focal mucinous differentiation. It is important that carcinomas with mixed histologic patterns be distinguished from pure mucinous carcinomas. Even though recent genetic evidence (discussed later in this chapter) shows that mixed mucinous carcinomas are closely related to pure mucinous carcinomas, mixed mucinous tumors are still best managed as infiltrating duct carcinomas not otherwise specified (NOS).
CLINICAL PRESENTATION
Incidence
When the diagnosis of mucinous carcinoma is restricted to tumors consisting of pure or nearly pure mucinous carcinoma, their incidence ranges from 1% to 2% of all breast carcinomas in most series.8,10,11,12,13,14,15,16 Interestingly, mucinous carcinomas constituted 5.2% of breast carcinomas treated at one center in Tanzania.17
Age
Mucinous carcinoma occurs throughout most of the age range of breast carcinoma. Most studies have reported the mean age of women with mucinous carcinoma to be older than that of patients with nonmucinous tumors.11,14,15,19,20,21,22,23 Di Saverio et al.21 evaluated Surveillance Epidemiology and End Results (SEER) data for 11,422 patients with pure mucinous carcinomas. The median and the mean age at diagnosis were 71 and 68.3 years (range 25 to 85), respectively, both significantly greater than for patients with invasive ductal carcinoma NOS (median and mean age, 61 years) (p < 0.01). Most patients with mucinous carcinoma (66.3%) were 65 years or older, 15.5% were between 55 and 65 years old, and 18.2% under age 55 years. In the Netherland Cancer Registry study,11 the age of patients with mucinous carcinoma was 70 years or greater in 2,053/3,482 (59%) cases, and between 50 and 69 years in 971/3,482 (28%) cases. Only 458/3,482 (13%) patients were younger than 50 years old. Komenaka et al.9 identified 65 patients (0.8%) with pure mucinous carcinoma among 7,676 women with breast carcinoma treated at a single institution. The age range at diagnosis was 13 to 93 years (mean, 67 years), and 85% of the patients were postmenopausal. Scopsi et al.14 reported that a significantly greater proportion of women with mucinous carcinoma were older than 50 years than was the case among patients with carcinoma having focal mucinous differentiation or nonmucinous carcinoma. Analysis of patients at the extremes of the age distribution for breast carcinoma revealed a striking difference because mucinous carcinoma constituted about 7% of carcinomas in women 75 years or older and only 1% among those younger than 35 years.24 No significant difference in the age distribution and median age of women with pure and mixed mucinous carcinoma has been found in a few studies,25,26,27 but in a series by Paramo et al.23 the mean age of patients with pure mucinous carcinoma was 75 years (range 59 to 90), whereas the mean age
of patients with mixed mucinous carcinoma was 65 years (range 35 to 89). This difference was reported to be statistically significant (p = 0.02).
of patients with mixed mucinous carcinoma was 65 years (range 35 to 89). This difference was reported to be statistically significant (p = 0.02).
Compared with series of mucinous carcinoma in Western countries, a series from Tanzania17 and two from South Korea27,28 have reported relatively younger mean age at diagnosis of mucinous carcinoma than for nonmucinous tumors, namely age 55 years for Tanzanian women and 4427 and 4528 years for Korean women.
As might be expected from the age distribution, most patients in the United States with mucinous carcinoma are postmenopausal.10,29 Two studies29,30 found that about 78% of postmenopausal patients with mucinous carcinoma never used hormone replacement therapy, and less than 30% reported using hormone replacement therapy prior to or at diagnosis. A decreased incidence of mucinous carcinoma was observed in women taking combined estrogen and progesterone replacement therapy,31 but there was no change in the incidence in women taking only estrogen-based replacement therapy. Work et al.30 documented an inverse association of mucinous carcinoma with the use of oral contraceptives, late age of menarche, and parity, but a positive association with late age at first birth.
Inflammation in the adipose tissue of women with high body mass index (BMI) has been linked to increased aromatase levels that may play a role in the pathogenesis of breast carcinoma.32 In one study,29 46.4% of women with mucinous carcinoma had a BMI greater than 26.6 kg/m2 versus 33.4% of women with invasive ductal carcinoma NOS, but the authors did not evaluate whether this result was significantly associated with mucinous carcinoma. Li et al.33 found no correlation between BMI and mucinous carcinoma, but they reported that height greater than 160 cm correlated with 2.5-fold increase in the relative risk (RR) of developing this tumor. In a Korean series,27 a family history of breast carcinoma was reported in 9% of patients with pure mucinous carcinoma and 6.1% of patients with mixed mucinous carcinoma. Another study33 found no association between mucinous carcinoma and having a first degree relative with breast carcinoma. No association of mucinous carcinoma and BRCA1 germline mutation has been documented.30 Lacroix-Triki et al.34 found no evidence of microsatellite instability (MSI) that is associated with Lynch syndrome in mammary mucinous carcinomas.
Ethnicity
Mucinous carcinoma is most frequent among Caucasian women.12,21,30,35 An analysis of 1973 to 2002 SEER data by Di Saverio et al.21 found that 85.2% women with mucinous carcinoma were White, 7% were African American, and 7.8% of other or unknown ethnicity. A subsequent analysis of 1992 to 2007 SEER data by Li et al.12 documented that 78.5% of women with mucinous carcinoma were non-Hispanic Whites, 7.1% African Americans, 9.1% Asian/Pacific Islanders, 4.1% Hispanic Whites, 0.7% American Indian/Alaska Native, and 0.4% of other ethnicity. Similar percentages were reported by Barkley et al.35 In a population-based study30 with data from California (United States), Ontario (Canada), and Melbourne (Australia), 54% of mucinous carcinomas occurred in Whites, 11% in Blacks, 11% in Hispanics, 19% in Asians, and 4% in other ethnic groups.
Mucinous carcinoma can also occur in men. Based on SEER data, the incidence of mucinous carcinoma in men was 0.5% between 1973 and 2002,21 but rose to 2% between 1985 and 2000.36 Burga et al.37 found 21 (2.8%) pure mucinous carcinomas and 26 (3.4%) mixed mucinous carcinomas in a series of 759 primary invasive carcinoma in men.
Clinical Presentation
The initial symptom of a pure mucinous carcinoma usually is a soft breast mass, but, since the introduction of widespread screening mammography, a substantial proportion of patients present with nonpalpable mammographic lesions.22,38,39 In one study,29 44.6% of 56 mucinous carcinomas were self-detected, 37.5% were detected at mammographic screening, and 17.9% were first identified at clinical examination. In another series,40 a palpable mass was the presenting symptom in 87% cases. Nipple discharge,22,40 Paget disease,41 and pain are uncommon. Fixation to the skin and chest wall occurs with large lesions. Palpation reveals a soft to moderately firm lesion. On reviewing patient records one rarely finds the “swish sign” mentioned. About half of mucinous carcinomas occur in the upper outer quadrant, and the other half is distributed in the remaining quadrants,21 with anatomic distribution not significantly different from that of other types of breast carcinoma. Mucinous carcinoma can arise in ectopic breast tissue at superficial sites such as the axilla or vulva that might be subject to needle biopsy.42,43 The differential diagnosis in these unusual locations will involve metastatic carcinoma from an extrinsic primary or mucinous carcinoma arising from sweat glands. It is necessary to document the presence of benign mammary glands to consider a diagnosis of mucinous carcinoma originating in ectopic breast tissue. The presence of ductal carcinoma in situ (DCIS) in this tissue will firmly establish the diagnosis of primary mucinous carcinoma in an ectopic site.
Imaging
Tumors with a high proportion of mucin production tend to be mammographically (Fig. 18.1A) and sonographically (Fig. 18.1B) lobulated or circumscribed.39,44,45,46,47 These lesions are likely to have a slow growth rate, determined by comparing serial mammograms and also low frequency of axillary nodal metastases. Only 37.5% of mucinous carcinomas in one series29 were first detected at mammographic screening. In another study,28 the sensitivity of mammogram for the detection of pure mucinous carcinoma was only 76.5% versus 100% for mixed mucinous carcinomas. In the series by Li et al.,12 31/39 (79.5%) pure mucinous carcinomas and 6/7 (86%) mixed mucinous carcinoma formed mammographically detected masses that were circumscribed and indistinct, respectively, in 36% and 28% of pure mucinous carcinomas, but showed equivalent features only in 14%
of mixed mucinous tumors. Two pure mucinous and one mixed mucinous carcinoma were mammographically occult. Mixed mucinous carcinomas typically have irregular margins mammographically because of fibrosis and an infiltrative growth pattern.48 A spiculated contour is associated with a lesser mucinous component and a higher frequency of lymph node metastases. Mammographically detected calcifications can occur in up to 40% of the tumors and involve the invasive portion of mucinous carcinomas in approximately 20% of cases.22,40,46,47,48,49 Calcifications constituted the only mammographic evidence of a pure mucinous carcinoma in only a handful of cases,40,45,50 and may be limited to associated DCIS or to a concurrent mucocele-like lesion (MLL).51
of mixed mucinous tumors. Two pure mucinous and one mixed mucinous carcinoma were mammographically occult. Mixed mucinous carcinomas typically have irregular margins mammographically because of fibrosis and an infiltrative growth pattern.48 A spiculated contour is associated with a lesser mucinous component and a higher frequency of lymph node metastases. Mammographically detected calcifications can occur in up to 40% of the tumors and involve the invasive portion of mucinous carcinomas in approximately 20% of cases.22,40,46,47,48,49 Calcifications constituted the only mammographic evidence of a pure mucinous carcinoma in only a handful of cases,40,45,50 and may be limited to associated DCIS or to a concurrent mucocele-like lesion (MLL).51
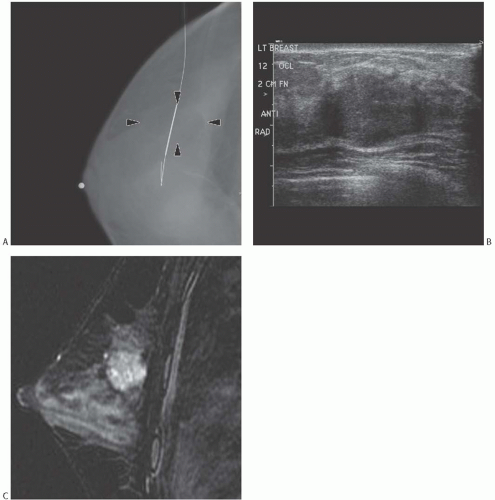 FIG. 18.1. Mucinous carcinoma, radiologic appearances. A: This mammographic image, postneedle localization, shows an irregular and heterogeneous 3 cm solid mass (arrows). The mass is localized by the thick portion of the wire. B: The sonographic image reveals an oval-shaped hypoechoic solid 1.5 cm mass with irregular borders. C: An MRI sagittal fat-suppressed T2-weighted image shows a lobulated T2-hyperintense rim-enhancing 2.3-cm mass. The surrounding breast is dense with moderate background enhancement. |
On ultrasound examination (Fig. 18.1B) mucinous carcinoma is isoechogenic to the breast fat,52 and may be difficult to detect. In one study,28 the sensitivity of ultrasound for the detection of pure mucinous carcinoma was 94.7%, whereas it was 100% for mixed mucinous carcinomas. In a series by Dhillon et al.,47 11/28 (39%) mammographically evident pure mucinous carcinomas were not seen on ultrasound. The tumor size ranged from 5 to 20 mm, with an average of 11 mm. A total of 13/34 (38%) pure mucinous carcinomas were not
recognized as abnormal when first encountered in a mammogram or at ultrasound examination, with a consequent delay in diagnosis. The authors, however, specified that the delayed diagnosis did not have clinical impact, as none of the patients had lymph node metastases at the time of surgical excision. On ultrasound, myxoid fibroadenoma and benign cystic lesions, as well as high-grade matrix-producing carcinoma and high-grade carcinoma with central acellular zone, sometimes resemble pure mucinous carcinoma.53
recognized as abnormal when first encountered in a mammogram or at ultrasound examination, with a consequent delay in diagnosis. The authors, however, specified that the delayed diagnosis did not have clinical impact, as none of the patients had lymph node metastases at the time of surgical excision. On ultrasound, myxoid fibroadenoma and benign cystic lesions, as well as high-grade matrix-producing carcinoma and high-grade carcinoma with central acellular zone, sometimes resemble pure mucinous carcinoma.53
In MRI imaging (Fig. 18.1C), pure mucinous carcinoma has a gradually enhancing contrast pattern and very high signal intensity on T2-weighted images.54,55 In a study by Monzawa et al.,56 pure mucinous carcinomas and mixed mucinous carcinomas had high signal intensity on T2-weighted images and the pattern of early phase enhancement varied with tumor cellularity and was more gradual in hypocellular tumors. The MRI characteristics of mucinous carcinomas and fibroadenomas are not distinctively different.57,58
Symptoms
The average duration of symptoms prior to biopsy and diagnosis tends to be 3 months or less, but some elderly patients who have large lesions may delay seeking treatment for considerably longer.7 One group of investigators observed that the majority of patients with tumors 4 cm or larger were older than 70 years.5
GROSS PATHOLOGY
Size
Mucinous carcinomas can range in size from less than 1 cm to more than 20 cm in diameter. Studies published prior to 1975 stressed the relatively large size of mucinous carcinomas,7,59,60 but this has not been observed in more recent reports.
In the study by Di Saverio et al.,21 mucinous carcinoma had a mean size 2.2 cm and median size 1.6 cm, with 83.2% of tumors measuring 3.0 cm or less. The size of the mucinous carcinoma was significantly smaller than the size of invasive ductal carcinoma NOS in the same period, and the same trend applied to nodal involvement.
Mucinous carcinomas were slightly smaller than invasive ductal carcinomas also in a study by Cao et al.22 Over half (56%) of the tumors measured 2 cm or less, 37.5% measured between 2 and 5 cm, and 4.9% were larger than 5 cm. The average size of mucinous carcinoma was 1.6 cm (range 0.1 to 6.0) in the series by Barkely et al.,35 and larger tumor size significantly correlated with lymph node involvement. The average size of pure mucinous carcinomas with no lymph node metastases was 1.5 cm versus 2.6 cm for tumors with nodal involvement. In the series by Bae et al.,27 pure mucinous carcinomas were T1 tumors in 55.6% of cases, T2 in 41.1%, T3 in 2.8%, and T4 in 0.5% of cases, compared with 45.3% T1, 47.2% T2, and 7.5% T3 mixed mucinous carcinomas.
A nationwide study of Danish patients with breast carcinoma found that only 16% of mucinous carcinomas were larger than 5 cm.8 In a series from Finland, a greater proportion of mixed (48%) than of pure (22%) mucinous carcinomas was larger than 5 cm,15 and a study from Japan stated that 53.6% of mucinous tumors measured 2.0 cm or less (T1) and 37.8% were 2.1 to 5.0 cm (T2).18 Fentiman et al.25 reported that pure mucinous tumors were significantly smaller (mean, 2.17 cm) than mixed mucinous tumors (mean, 3.25 cm). Ranade et al.26 also found that the mean tumor size of pure mucinous carcinomas was 1.65 cm and 2.5 cm for mixed mucinous tumors,26 but Diab et al.20 did not find a significant difference in size between mucinous and nonmucinous carcinomas.
Out of 19 patients with pure mucinous carcinoma reported by Paramo et al.,23 6 (31%) had T1b tumors, 8 (42%) were T1c, 2 (11%) with tumors spanning between 2 and 3 cm, and 3 (16%) had tumors larger than 3 cm. In the group of 41 mixed mucinous carcinomas, 20% were T1b, 42% were T1c, 27% were T2 tumors spanning between 2 and 3 cm, 3 (7%) were T2 tumors larger than 3 cm, and 2 were T3 tumors. The size differences were not statistically significant.
Gross Characteristics
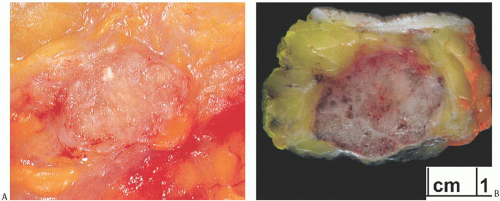
On palpation of the excised tumor, the consistency of mucinous carcinoma varies depending upon the amount of fibrous stroma in the lesion. When stroma is sparse, the tumor feels soft and gelatinous. The cut surface is typically moist and glistening, even in relatively fibrotic tumors (Fig. 18.2). Most mucinous carcinomas have a circumscribed gross margin, which may be accentuated by a peripheral red-topurple zone of congested parenchyma. Cystic degeneration has been reported in relatively large tumors.
MICROSCOPIC PATHOLOGY
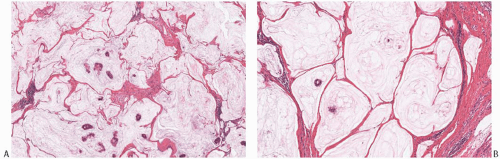
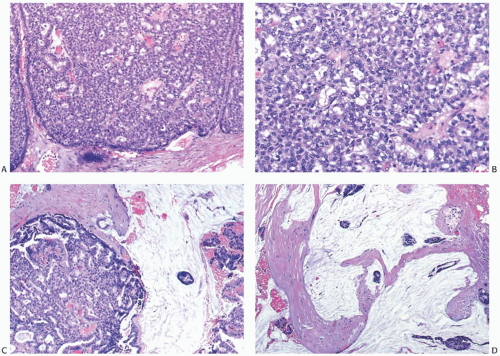
The hallmark of mucinous carcinomas is the accumulation of abundant extracellular mucin around the invasive tumor cells (Fig. 18.3). The relative proportions of mucin and neoplastic epithelium vary from one case to another, but the distribution in any one tumor is fairly constant (Figs. 18.3 and 18.4). Multiple sections may be required to detect carcinoma cells in a tumor composed almost entirely of extracellular mucin (Fig. 18.5). In one study, the proportion of extracellular mucin in tumors classified as pure mucinous carcinomas varied from slightly less than 70% to nearly 100%, with a mean percentage of 83.5 ± 14.3.18 Infiltrating duct carcinomas with a mucinous component had a lower mean proportion of extracellular mucin (68.3% ± 16.6%), with the distribution ranging from 32% to 97%. In practice, the diagnosis of pure mucinous carcinoma is reserved for tumors in which more of 90% of the invasive component is admixed with stromal mucin. Any tumor with stromal mucin in 50% to 90% of the lesion is best classified as mixed mucinous carcinoma (Figs. 18.6 and 18.7). If stromal mucin
represents less than 50% of the tumor mass, the component should be mentioned in the diagnosis, but no specific designation applies. The abundant extracellular mucin in pure mucinous carcinomas may constitute an obstacle to tumor vascularization, lymphovascular permeation, and lymph node metastasis,61 and account for the relatively good prognosis of these tumors. The majority of pure mucinous carcinomas are well to moderately differentiated. It is extremely rare for a mucinous carcinoma to have high nuclear grade,26 and in such cases, this information should be clearly stated and emphasized in the diagnostic report, as the clinical behavior may not be as indolent as for usual pure mucinous carcinomas. In the SEER data-based series (11,422 cases) by Di Saverio et al.,21 53% of the tumors were well differentiated, 38% were moderately differentiated, and the remaining 9% were poorly differentiated or anaplastic.
represents less than 50% of the tumor mass, the component should be mentioned in the diagnosis, but no specific designation applies. The abundant extracellular mucin in pure mucinous carcinomas may constitute an obstacle to tumor vascularization, lymphovascular permeation, and lymph node metastasis,61 and account for the relatively good prognosis of these tumors. The majority of pure mucinous carcinomas are well to moderately differentiated. It is extremely rare for a mucinous carcinoma to have high nuclear grade,26 and in such cases, this information should be clearly stated and emphasized in the diagnostic report, as the clinical behavior may not be as indolent as for usual pure mucinous carcinomas. In the SEER data-based series (11,422 cases) by Di Saverio et al.,21 53% of the tumors were well differentiated, 38% were moderately differentiated, and the remaining 9% were poorly differentiated or anaplastic.
In mucinous carcinomas, the tumor cells are arranged in a variety of patterns (Figs. 18.4 and 18.8). Usually the epithelial pattern duplicates the structure of the associated DCIS. These configurations include tumor cells in strands, alveolar nests, and papillary clusters, as well as larger sheets of cells that may have cribriform areas or focal comedonecrosis. Tubule and gland formation are uncommon.
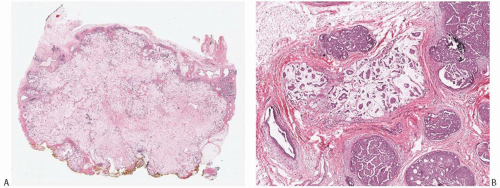 FIG. 18.3. Pure mucinous carcinoma. A: Low-power view of a pure mucinous carcinoma. The carcinoma is relatively hypocellular, and the neoplastic epithelial clusters are admixed with mucin throughout. B: This 3 mm pure mucinous carcinoma consists of clusters of neoplastic epithelial cells within mucin pools. Cribriform and papillary DCIS with intermediate nuclear grade and calcifications are present. Note the absence of extracellular mucin in the DCIS. |
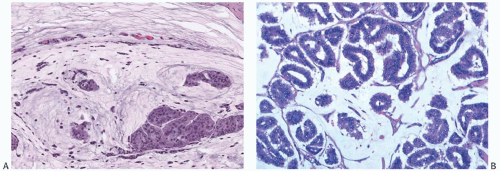 FIG. 18.4. Mucinous carcinoma. A: A moderately cellular carcinoma. Note the presence of capillaries in the mucin. B: A very cellular carcinoma with a glandular pattern. |
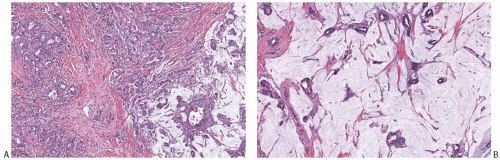
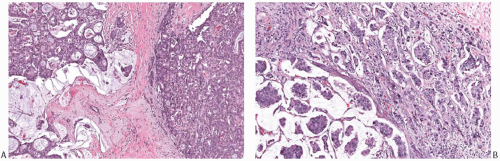 FIG. 18.7. Infiltrating ductal carcinoma with mucinous features. A,B: Two infiltrating ductal carcinomas that have limited, discrete areas of mucinous growth. |
The margin of a mucinous carcinoma is determined by the extent of the mucinous component, even if no epithelial cells are seen in it. The periphery of the tumor is characterized by a pushing border in more than 70% of cases45 (Figs. 18.3, 18.9, and 18.10). Some of these tumors have irregular or knobby contours formed by protrusions of the neoplasm into the breast parenchyma (Fig. 18.10). When assessing the margins of excision, it is important to look for transected protrusions that may be obscured by cautery artifact or blend with fat. When evaluating the margin status of a surgical specimen from a patient with known mucinous carcinoma, the presence of mucin at ink should be interpreted as tumor at margin, even if the transected mucin is devoid of neoplastic cells, as long as artifactual contamination can be excluded.
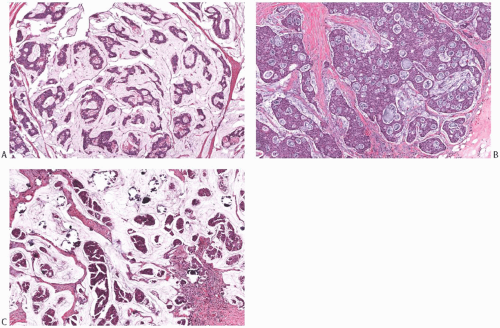 FIG. 18.8. Mucinous carcinoma, different patterns. A: Trabecular and cribriform patterns. B: Solid and cribriform patterns. C: Papillary mucinous carcinoma with calcifications. |
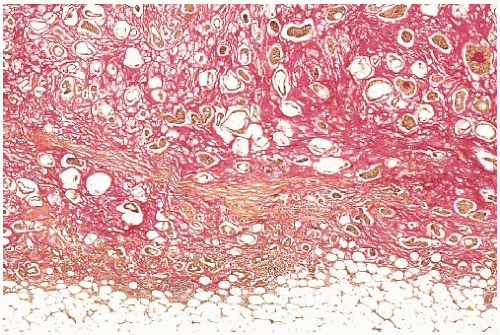 FIG. 18.9. Mucinous carcinoma. The mucin stained a rose color has a distinct and pushing border. Clusters of carcinoma cells are present in the mucin (mucicarmine stain). |
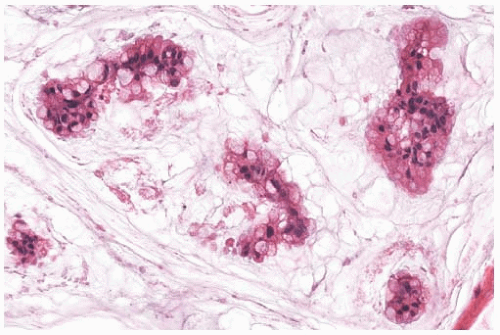
It is very difficult to recognize lymphatic tumor emboli in mucinous carcinoma. Clusters of carcinoma cells suspended in mucin often have an appearance that resembles intralymphatic carcinoma (Fig. 18.11). When the diagnosis of such foci is uncertain, a stain for mucin may be helpful, because the material surrounding the carcinoma cells will be clearly stained in mucinous carcinoma but it tends to be weakly reactive in lymphatic tumor emboli. However, this finding is not definitive, as mucin sometimes can be found admixed with clusters of carcinoma within vascular spaces (Fig. 18.12). Immunostains for vascular endothelial markers such as CD31, CD34, factor VIII, and D2-40 may also be helpful.
Among mucinous carcinomas of the breast, calcifications are most often found in tumors with papillary (Fig. 18.8C) or comedo epithelial patterns. The calcifications tend to be coarse and irregular. Mucinous carcinoma with micropapillary morphology often contains psammomatous calcifications.48,50,62,63,64,65
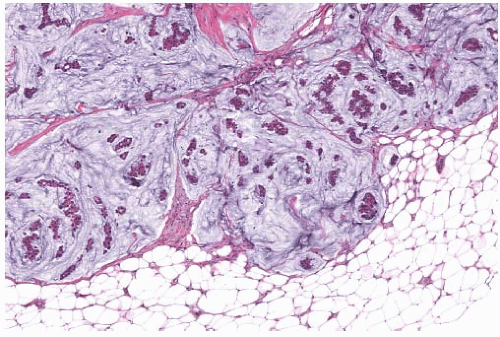 FIG. 18.10. Mucinous carcinoma. This is a mucinous carcinoma with peripheral knobby protrusions in the adjacent fatty breast tissue. |
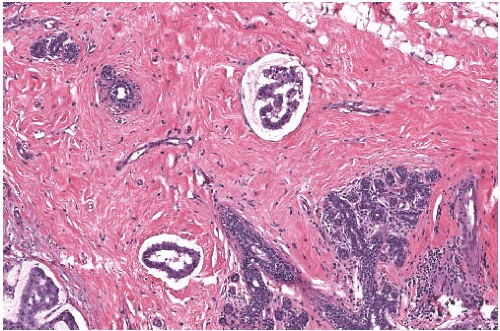 FIG. 18.11. Mucinous carcinoma. These discrete, sharply defined foci of invasive mucinous carcinoma resemble tumor in vascular spaces. |
Type A, Type B, and Type AB Mucinous Carcinomas
Capella et al.66 presented criteria for the subclassification of mucinous carcinoma on the basis of the epithelial growth pattern and some associated features. They described two principal types (A and B) and an intermediate category (AB). By definition, at least 33% of each tumor consisted of extracellular mucin, but, overall, mucin was more abundant in type A (Fig. 18.13A) than in type B lesions (Fig. 18.13B). Type A tumors had epithelium distributed in “trabeculae and ribbons or festoons.” “Clumps” of cells, uncommon in type A tumors, were the characteristic growth pattern of type B lesions. Cribriform areas were seen in both tumor types. Intracytoplasmic mucin was more abundant in type B lesions, and the cells in these tumors tended to have more granular cytoplasm than in type A carcinomas. Cells with “foamy” cytoplasm were detected in a minority of type A tumors and in none of the type B group. Ten of 14 type B tumors contained
argyrophilic granules detected with Grimelius and Bodian stains, whereas all 15 type A tumors had no detectable argyrophilic granules (Fig. 18.14). A statistically significant difference was found in the age distribution of patients with type A and B lesions, with the former group tending to be younger at diagnosis. Type AB tumors constituted 20% of the cases studied and were described as having “indeterminate” features or features “indicative of transitional forms between the two major groups,” but little information was given about these cases. The authors concluded that the type A lesions corresponded to carcinomas that were ordinarily regarded as classical pure mucinous carcinoma. It was recommended that type B tumors be regarded as a variant of mucinous carcinoma with endocrine differentiation.
argyrophilic granules detected with Grimelius and Bodian stains, whereas all 15 type A tumors had no detectable argyrophilic granules (Fig. 18.14). A statistically significant difference was found in the age distribution of patients with type A and B lesions, with the former group tending to be younger at diagnosis. Type AB tumors constituted 20% of the cases studied and were described as having “indeterminate” features or features “indicative of transitional forms between the two major groups,” but little information was given about these cases. The authors concluded that the type A lesions corresponded to carcinomas that were ordinarily regarded as classical pure mucinous carcinoma. It was recommended that type B tumors be regarded as a variant of mucinous carcinoma with endocrine differentiation.
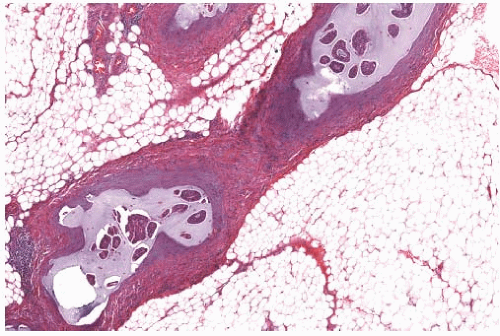 FIG. 18.12. Mucinous carcinoma in vascular spaces. Mucin is admixed with tumor emboli in vascular spaces of axillary soft tissue. |
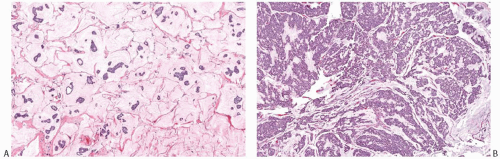 FIG. 18.13. Mucinous carcinoma, types A and B. A: Type A tumors such as this have abundant extracellular mucin. B: Type B tumors are hypercellular. |
Scopsi et al.14 confirmed the common occurrence of argyrophilic granules in type B carcinomas. The presence or absence of argyrophilia was not significantly related to age, menstrual status, tumor size, or axillary nodal status. Classification as type A or type B did not prove to be prognostically significant.
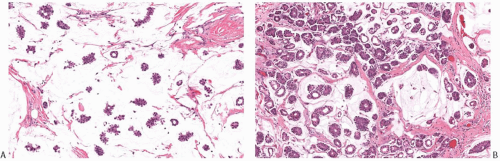
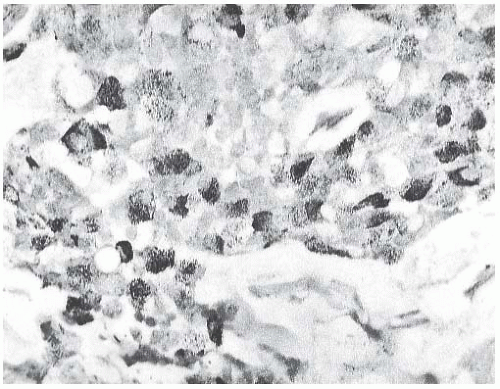 FIG. 18.14. Mucinous carcinoma. Clusters of fine black argyrophilic granules are demonstrated with the Grimelius stain in this island of solid carcinoma. |
Ranade et al.26 compared the characteristics of 37 type A and 8 type B pure mucinous carcinomas. The mean age of patients with type A tumors was 75 years. Type A mucinous carcinomas had a mean size 1.4 cm. Histologically, 65% of type A tumors were well differentiated, 35% were moderately differentiated, and none had high-grade morphology. Lymphovascular invasion was detected in 1/37 (3%) cases. One of three patients with type A carcinoma and lymph node metastases was 44 years old and the other two were 46 years old. Two of the three tumors with lymph node involvement had a micropapillary pattern. Two of 37 (5.5%) type A tumors were human epidermal growth factor 2 (HER2) positive. The mean age of patients with type B carcinoma was 55 years. Type B mucinous carcinomas had a mean size of 1.9 cm. Histologically, 50% type B tumors were moderately differentiated, 25% well differentiated, and 25% poorly differentiated. Lymphovascular invasion and lymph node involvement were detected in 2/8 (25%) patients, one of whom was 44 years old and the other 82 years old. Two of eight (25%) type B tumors were HER2 positive. These findings suggest that type A tumors may have more favorable characteristics than type B tumors, but this hypothesis needs confirmation in larger studies.
The morphologic distinction between type A and B mucinous carcinoma has a strong correlation with genetic subtype (see section on genetics and molecular alterations in this chapter), but at present, the designation of type A or B morphology has no clinical implications, and is rarely mentioned in routine diagnostic reports.
Micropapillary Variant of Pure Mucinous Carcinoma
A micropapillary variant of pure mucinous carcinoma has been described (Fig. 18.15).26,62,67,69 The micropapillae are arranged in small clusters that are tightly cohesive or have
a central open space with a resulting ring-like configuration. The tumor clusters are surrounded by a clear space filled with mucin. Epithelial membrane antigen (EMA) decorates the outer surface of the micropapillae, confirming the everted polarity of the epithelium,26,67,68 akin to invasive micropapillary carcinoma. A micropapillary component was recognized in 66.6%,68 35%,67 and 20%26 of pure mucinous carcinomas in three separate series. In a series of 102 pure mucinous carcinomas, Shet and Chinoy68 had 20 cases in which a mucinous micropapillary component coexisted with invasive micropapillary carcinoma. In the series by Ranade et al.,26 pure mucinous carcinomas with and without micropapillary component had similar average size (1.7 and 1.65 cm, respectively), but patients with a micropapillary component were younger than those without (47 vs. 60 years, respectively). Three of the five (60%) carcinomas with lymph node metastases in this series had a micropapillary component, whereas only 14% of pure mucinous carcinomas without lymph node involvement showed micropapillary foci.26 In another series,69 lymphovascular invasion was present in 9/15 (60%) mucinous micropapillary carcinomas. Lymph node metastases were found in 33% of all cases. One of 13 patients with follow-up information developed a chest wall recurrence 9 months after mastectomy.
a central open space with a resulting ring-like configuration. The tumor clusters are surrounded by a clear space filled with mucin. Epithelial membrane antigen (EMA) decorates the outer surface of the micropapillae, confirming the everted polarity of the epithelium,26,67,68 akin to invasive micropapillary carcinoma. A micropapillary component was recognized in 66.6%,68 35%,67 and 20%26 of pure mucinous carcinomas in three separate series. In a series of 102 pure mucinous carcinomas, Shet and Chinoy68 had 20 cases in which a mucinous micropapillary component coexisted with invasive micropapillary carcinoma. In the series by Ranade et al.,26 pure mucinous carcinomas with and without micropapillary component had similar average size (1.7 and 1.65 cm, respectively), but patients with a micropapillary component were younger than those without (47 vs. 60 years, respectively). Three of the five (60%) carcinomas with lymph node metastases in this series had a micropapillary component, whereas only 14% of pure mucinous carcinomas without lymph node involvement showed micropapillary foci.26 In another series,69 lymphovascular invasion was present in 9/15 (60%) mucinous micropapillary carcinomas. Lymph node metastases were found in 33% of all cases. One of 13 patients with follow-up information developed a chest wall recurrence 9 months after mastectomy.
Signet Ring Variant of Pure Mucinous Carcinoma
The pathognomonic feature of signet ring cells is abundant intracytoplasmic mucin, either concentrated within an intracytoplasmic vacuole or uniformly dispersed throughout the cytoplasm, resulting in indentation of the nucleus and displacement to one side of the cell. Carcinomas with abundant extracellular mucin rarely also have signet ring cell morphology (Fig. 18.16). On the other hand, the signet ring cell variant of infiltrating lobular carcinoma rarely has an extracellular mucinous component, and it is best considered as a mucinous variant of infiltrating lobular carcinoma,70 not as a mucinous carcinoma. Signet ring cells admixed with mucin are often found in pure mucinous carcinomas with neuroendocrine features (type B), and can consist of single cells or, more commonly, large solid clusters. In their series of 102 pure mucinous carcinomas, Shet and Chinoy68 identified seven solid and papillary carcinomas composed of signet ring cells arranged in solid epithelial clusters admixed with mucin. An example of this lesion is probably the case reported by Kuroda et al.71
Mucinous Carcinoma Associated with Solid and Papillary Carcinoma
Mucinous carcinoma may also arise from solid papillary carcinoma (Fig. 18.17). These tumors usually are pure mucinous carcinomas with type B morphology and show neuroendocrine differentiation or have neuroendocrine features. They overlap morphologically with carcinomas with neuroendocrine differentiation (see Chapter 20). Genetic evidence also supports a close relationship between the two entities.72,73
Cystic Papillary Mucinous Carcinoma (Mucinous Cystadenocarcinoma)
One of the most infrequent variants of mucin-producing carcinoma is a cystic type of papillary mucinous carcinoma (Fig. 18.18). It is composed of multiple cysts distended by mucinous secretion and lined by micropapillary, papillary, and cribriform carcinoma. The mucinous epithelium can have blandly atypical nuclei or show more nuclear atypia, often accompanied by intracytoplasmic mucin depletion.74,75,76,77,78,79,80,81,82 All reported cases have occurred in women, ranging in age from 4183 to 96 years old.75 Many of the case reports have involved women in Asian countries.75,76,78,80,81,83,84 Lack of myoepithelial lining around the mucin-filled cysts of cystic papillary mucinous carcinoma has been documented,74,78,82 a finding that supports an invasive process. Rarely, areas of invasive ductal carcinoma NOS76,78 as well as foci of sarcomatoid metaplasia74 have been observed admixed with these tumors, but the usual morphology of pure mucinous carcinoma has not been documented in this setting. Focal DCIS has also been found.74,76,78,82 Squamoid differentiation has been reported in a few cases.74,75
Because cystic papillary mucinous carcinomas of the breast are estrogen receptor (ER) and progesterone receptor (PR) negative74,75,76,77,78,80,81,83,84 (see section on immunohistochemistry [IHC]), whenever the differential diagnosis of cystic papillary mucinous carcinoma is considered and no adjacent DCIS and/or invasive mammary carcinoma NOS are identified, careful clinical and radiologic correlation and thorough review of the patient’s prior medical history are recommended to rule out metastasis from an ovarian or colorectal mucinous carcinoma. Eleven patients with cystic papillary mucinous carcinoma described by Komaki et al.85 had a relatively lower average age at diagnosis (41 years) than is typical for mucinous carcinoma. None of the patients had axillary nodal metastases when treated by mastectomy, and they remained disease-free for an average of nearly 10 years. Mucinous cystadenocarcinoma might be derived from a metaplasia of ordinary DCIS, but its pathogenesis and biologic behavior remain unclear. Despite their invasive nature and large size
at presentation, cystic papillary mucinous carcinomas of the breast appear to be associated with a relatively good prognosis, and only few patients had lymph node involvement.74,75,83 Metastatic carcinoma arising from these tumors usually has morphology similar to that of the primary tumor.
at presentation, cystic papillary mucinous carcinomas of the breast appear to be associated with a relatively good prognosis, and only few patients had lymph node involvement.74,75,83 Metastatic carcinoma arising from these tumors usually has morphology similar to that of the primary tumor.
Ductal Carcinoma In Situ
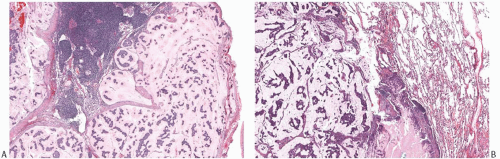
DCIS is found associated with 60% to 75% of the lesions, and is generally located at the tumor periphery.25 The intraductal component has any of the conventional patterns of DCIS (cribriform, papillary, micropapillary, and solid patterns). Marked zonal necrosis is present rarely. Occasionally, prominent mucin is present in the lumen of the intraductal component (Fig. 18.19), and one can find transitions from intraductal to invasive mucinous carcinoma (Fig. 18.19). DCIS was present in 37/40 (92.5%) mucinous carcinomas in one series.86 It ranged from focal to extensive and was cribriform in 24/37 (65%) cases, solid in 20/37 (54%), micropapillary in 11/37 (30%), and flat in 4/37 (11%). DCIS with necrosis was present in 11/37 (30%) cases. The nuclear grade was low in 12/37 (32%) cases, intermediate in 21/37 (57%), and high in 4/37 (11%) cases. Intraluminal mucin was identified in 32/37 (86%) DCIS cases and contained blood vessels in 26/37 (70%). The DCIS patterns most frequently associated with neovascularization of the intraluminal mucin were solid and cribriform. The high frequency of mucin neovascularization in the DCIS associated with mucinous carcinoma led the authors of the study to speculate that it might constitute an intermediate step toward stromal invasion, whereby the tumor cells first invade into the mucinous and vascularized stroma that they have induced and then into the surrounding fibroconnective tissue. However, in the absence of overt invasion, neovascularization of the mucin associated with DCIS should not be interpreted as evidence of tumor invasion (Fig. 18.20). A minority of mucinous carcinomas do not have detectable DCIS. These tend to be larger tumors, but rarely one encounters a pure mucinous carcinoma smaller than 2 cm with no apparent DCIS, sometimes arising in a MLL.
A diagnostic problem arises in patients who have only DCIS when extravasated mucin is present in the adjacent stroma. Extravasation of mucin from DCIS can be caused by a prior procedure or trauma or it may occur spontaneously. Mucin might also artifactually be extruded into the stroma during handling of a tissue specimen. For these reasons, the finding of extravasated mucin devoid of carcinoma cells does not necessarily always represent evidence of an invasive mucinous carcinoma. In these cases, it is necessary to obtain multiple recuts, and it is advisable to do a cytokeratin (CK) stain to determine whether epithelial cells are present in the mucin and to distinguish them from histiocytes. Immunoperoxidase stains for myoepithelial markers, such as calponin or p63, may highlight myoepithelial cells admixed with the detached clusters or present along the wall of ducts or lobules stripped of epithelium. However, lack of myoepithelium is not sufficient evidence of stromal invasion, especially if mucin and epithelial clusters are admixed with stromal changes secondary to a prior procedure. If carcinoma cells are found in the mucin, a diagnosis of invasive mucinous carcinoma is appropriate, unless there is compelling evidence to consider the alternative possibility of epithelial displacement, possibly secondary to a prior procedure. Most of the time, the mucin admixed with invasive carcinoma will also have a rounded to bulbous outline, neovascularization, and admixed inflammatory cells and fibroblasts.
Morphology of Metastases of Pure Mucinous and Mixed Mucinous Carcinoma
Axillary or systemic metastases (Fig. 18.21) that arise from mucinous carcinoma of the breast usually have the histologic characteristics of the primary tumor. Axillary metastases from tumors with mixed histology often resemble the nonmucinous component. Pure mucinous carcinomas occasionally can also have nonmucinous metastases.25 The
distribution of sites of disseminated metastases does not differ from that of other types of ductal carcinoma, but an unusual fatal complication is cerebral infarction caused by mucin embolism.87,88 The lymph node metastases of pure mucinous and micropapillary mucinous carcinoma are histologically similar to the primary tumor62, but pure micropapillary metastases occur69.
distribution of sites of disseminated metastases does not differ from that of other types of ductal carcinoma, but an unusual fatal complication is cerebral infarction caused by mucin embolism.87,88 The lymph node metastases of pure mucinous and micropapillary mucinous carcinoma are histologically similar to the primary tumor62, but pure micropapillary metastases occur69.
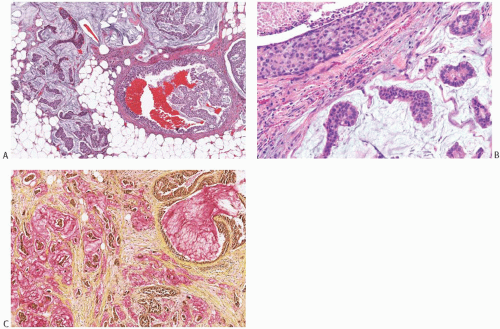 FIG. 18.19. Intraductal carcinoma in mucinous carcinoma. A: A duct containing DCIS and mucin (lower right) is present next to invasive mucinous carcinoma. B: DCIS with central necrosis (upper left) near invasive mucinous carcinoma. Intracellular mucin is evident in the DCIS cells C: Mucin stained pink is present in the lumen of the DCIS (upper right corner) and in the invasive carcinoma (mucicarmine stain). |
MUCOCELE-LIKE LESIONS
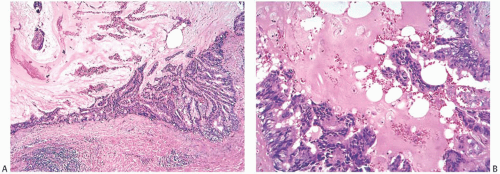
MLL, an entity first described and named by Rosen in 1986,51 is composed of mucin-containing cysts that tend to rupture and discharge the secretion into the adjacent stroma (Fig. 18.22). The resultant picture resembles the mucocele of minor salivary gland origin found in the oral cavity. It is important to note that the term “mucocele-like lesion” is descriptive and does not have specific implications regarding the biology of the lesion, which is dictated by the nature of the epithelium lining the cyst wall. Therefore, the final diagnostic report of a MLL needs to indicate whether the epithelium is benign, atypical, or frankly neoplastic. Some MLLs of the breast present as palpable tumors that are well circumscribed, lobulated lesions on mammography (Fig. 18.23), whereas others constitute incidental microscopic findings in excision specimen for a different lesion. An increasing number of small, nonpalpable, MLLs are detected by mammography alone. Mammography reveals a nodular lesion with or without calcifications, or clustered calcifications without a mass.89,90,91 Ultrasonography shows a hypoechoic, round or lobulated, solid or cystic tumor, sometimes with an ill-defined margin.92,93 Multiple aggregated cysts are evident grossly containing viscous, often transparent, mucinous material (Fig. 18.24).
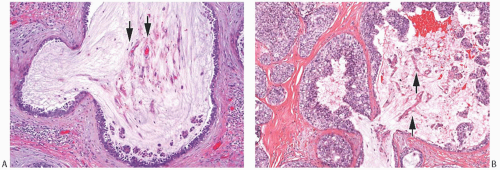 FIG. 18.20. Intraductal mucinous carcinoma. A,B: Neovascularization (arrows) of the mucin is present within the lumen of the DCIS. Papillary clusters of carcinoma cells are present in the mucin. |
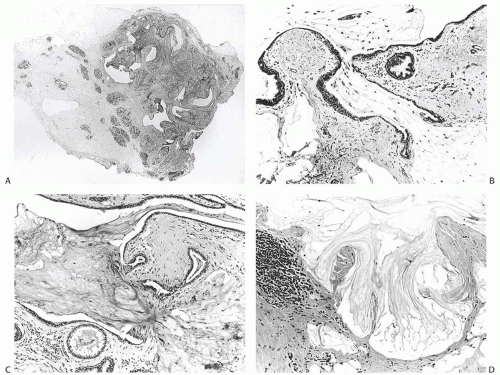 FIG. 18.22. Mucocele-like lesion. A: A whole-mount histologic section of a relatively small MLL. The dark oval foci to the left of the lesion are normal lobules. B,C: Two examples of ruptured cysts with mucin extruded into the surrounding stroma. Hyperplasia is evident in small ducts. Folding of epithelium toward the cyst of origin rather than into the stroma at the point of rupture is a typical finding. D: Pools of extruded mucin in fibrous stroma with a focal lymphocytic reaction. (Reproduced from Rosen PP. Mucocele-like tumors of the breast. Am J Surg Pathol 1986;10:464-469.) |
The epithelium lining the ducts in the typical mammary MLL is largely flat, attenuated or low cuboidal (Fig. 18.25), but low columnar and minor papillary elements may be present or the epithelium may show a spectrum of proliferative changes ranging from hyperplasia to atypical ductal hyperplasia (ADH) (Fig. 18.26) to DCIS.51,94,95 The distinction between mucinous carcinoma and MLL




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree