Modified Radical Mastectomy and Radical Mastectomy
Kirby I. Bland
A. History of Breast Cancer Surgery
In the second century AD, Galen, on his classical clinical observation of a breast carcinoma, said: “We have often seen in the breast a tumor exactly resembling the animal the crab. Just as the crab has legs on both sides of his body, so in this disease the veins extending out from the unnatural growth take the shape of a crab’s legs. We have cured this disease in its early stages, but after it has reached a large size, no one has cured it.” Beginning with Morgagni, surgical resections were more frequently undertaken, including some early attempts at mastectomy and axillary dissection. In the 18th century, le Dran incorrectly postulated that breast cancer was a local disease that spread by way of lymph vessels to axillary lymph nodes. When he operated on a woman with breast cancer, he routinely removed any enlarged axillary lymph nodes.
In 1867, C.H. Moore, of the Middlesex Hospital, London reemphasized complete resection of the breast for cancer and stated that palpable axillary lymph nodes should also be removed. In a presentation before the British Medical Association in 1877, Banks supported Moore’s concepts and advocated the resection of axillary lymph nodes even when palpable lymphadenopathy was not evident; recognizing that occult involvement of axillary lymph nodes was frequently present. In 1894, Halsted and Meyer reported their operations for treatment of breast cancer. By demonstrating superior locoregional control rates after radical resection, these surgeons established radical mastectomy (RM) as state of the art for that era. Both Halsted and Meyer advocated complete dissection of axillary lymph node levels I to III. Resection of the long thoracic nerve and the thoracodorsal neurovascular bundle with the axillary contents was routine. This technical maneuver contributed significantly to the surgical management of the disease.
However, in 1943, Haagensen and Stout described the grave signs of breast cancer, which included (a) edema of the skin of the breast; (b) skin ulceration; (c) chest wall fixation; (d) an axillary lymph node greater than 2.5 cm in diameter; and (e) fixed axillary lymph nodes. Women with two or more signs had a 42% local recurrence rate and only a 2% 5-year disease-free survival. Based on the findings, they declared that women with grave signs were beyond cure by radical surgery. Approximately 25% of women
were excluded from surgery based on the criteria of inoperability. Presently, with comprehensive mammography screening, approximately 10% of women are found to have advanced breast cancers.
were excluded from surgery based on the criteria of inoperability. Presently, with comprehensive mammography screening, approximately 10% of women are found to have advanced breast cancers.
A technical and aesthetic advance was proposed in 1948, when Patey and Dyson of the Middlesex Hospital, London, advocated “modified radical” mastectomy for the management of advanced operable breast cancer. The technique espoused by these surgeons included removal of the breast and axillary lymph nodes with preservation of the pectoralis major muscle. They showed that removal of the pectoralis minor muscle allowed access to and clearance of axillary lymph node levels I to III (Patey modification). Today, the modification is frequently limited to severance of the origin of the pectoralis major muscle at the coracoid process of the scapula. Subsequent to the description of the Patey modification, Madden advocated a modified RM that preserved both the pectoralis major and minor muscles even though this approach prevented complete dissection of the apical (level III) axillary lymph nodes.
With familiarity and experience in performance of the technique, by the 1980s, the surgical procedure most frequently used by American surgeons for breast cancer was modified RM. The transition from the Halsted RM to the modified RM acknowledged that (a) extirpation of the pectoralis major muscle was not essential for locoregional control in stage I and stage II breast cancer and (b) neither modified RM nor Halsted RM consistently achieved locoregional control of stage III breast cancer. The National Surgical Adjuvant Breast and Bowel Project B-04 (NSABP B-04) conducted by Bernard Fisher and coinvestigators thereafter compared local and regional treatments of breast cancer. Life table estimates were obtained for 1,665 women enrolled and followed for a mean of 120 months. This study randomized clinically node-negative women into three groups: (a) Halsted RM; (b) total mastectomy plus radiation therapy (TM+RT); and (c) total mastectomy alone (TM). Clinically node-positive women were treated with RM or TM+RT. After a median follow-up of 10 years, there were no differences in survival between the three groups of node-negative women or between the two groups of node-positive women.
Other prospective clinical trials comparing Halsted RM to the modified RM were the Manchester Trial, reported by Turner and colleagues, and the University of Alabama Trial, reported by Maddox and colleagues. In both studies, the type of surgical procedure did not influence recurrence rates for stage I and stage II breast cancer patients. Criteria for accrual to the Alabama Breast Cancer Project (1975 to 1978) included T1 to T3 breast cancers (Table 1) with absence of clinically apparent distant metastases. Patients received a radical or a modified RM. Node-positive patients received adjuvant cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy or adjuvant melphalan. After a median follow-up of 15 years, neither type of surgery nor type of chemotherapy was shown to affect locoregional disease-free or overall survival. Since the 1970s, considerable progress has been made in the integration of surgery, radiation therapy, and chemotherapy to control locoregional disease, to enhance survival, and to increase the possibility of breast conservation. Locoregional control is now achieved for nearly 80% of women with advanced breast cancers.
With the diagnosis of breast cancer indications, the type of therapy offered to a breast cancer patient is determined by the stage of the disease (Table 2). Survival rates for women diagnosed with breast cancer between 1983 and 1987 have been calculated based on Surveillance, Epidemiology, and End Results (SEER) Program data. Five-year survival for stage I patients is 94%, for stage IIa, 85%, and for stage IIb, 70%, while for stage IIIa patients 5-year survival is 52%, for stage IIIb, 48%, and for stage IV (distant disease), 18%.
Women At High Risk for Breast Cancer
Retrospective studies of women at high risk for breast cancer have found that prophylactic mastectomy can reduce their risk by greater than 90%. A study involving women who were carriers of BRCA-1 or BRCA-2 mutations found that the benefit of prophylactic mastectomy differed according to the breast cancer risk conferred by the individual mutations. For women with an estimated lifetime risk of 40% (approximately four times the population risk), prophylactic mastectomy added almost 3 years of life, whereas for women with an estimated lifetime risk of 85%, prophylactic mastectomy added greater than 5 years to survival based on genetic/familial risk of disease.
Variants of in Situ Breast Cancer (Stage 0)
Both lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS) may be pathologically difficult to distinguish from atypical hyperplasia or from cancers with early invasion. Expert pathological review is required in all cases. Since LCIS is considered a marker for increased risk rather than an inevitable precursor of invasive disease, the current treatment of LCIS is observation with or without antiestrogen therapy. The goal of treatment is to prevent or detect at an early stage the invasive cancer that subsequently develops in 25% to 35% of these women. Women with multifocal DCIS that is not amenable to lumpectomy and women with multicentric disease (involvement of two or more quadrants of the breast) require mastectomy. For women with limited disease, lumpectomy and radiation therapy is recommended. Sentinel lymph node biopsy is recommended for women with (a) multifocal DCIS; (b) multicentric DCIS; and (c) DCIS with comedonecrosis and tumor size ≥2.5 cm. In all cases, antiestrogen therapy is considered.
Early Invasive Breast Cancer (Stage I, Iia, or Iib)
The large, randomized prospective trial (NSABP B-06) compared TM with lumpectomy with or without radiation therapy for the treatment of stage I and II breast cancer. After 12 and 20 years of follow-up, the disease-free, distant disease-free, and overall survival rates for lumpectomy with or without radiation therapy were statistically similar to those observed after TM. However, the incidence of ipsilateral breast cancer recurrence (in breast recurrence) was higher in the lumpectomy group not receiving radiation therapy. The observations from this trial supported the use of lumpectomy and radiation in the treatment of stage I and II breast cancer. Currently, (a) breast conservation (lumpectomy and radiation therapy with sentinel lymph node biopsy and/or axillary lymph node dissection) or (b) mastectomy with sentinel lymph node biopsy and/or axillary lymph node dissection should be the recommended treatments for stage I and II breast cancer. Breast conservation is considered for all patients because of the important cosmetic and functional advantages. Relative contraindications to breast conservation therapy include (a) prior radiation therapy to the breast or chest wall; (b) involved surgical margins or unknown margin status following
reexcision; (c) multicentric disease; and (d) scleroderma or other connective tissue disorders. In all cases, axillary lymphadenopathy or metastatic disease in a sentinel axillary lymph node necessitates an axillary lymph node dissection. In all cases with tumor size >0.5 cm, adjuvant chemotherapy and/or antiestrogen therapy is considered.
reexcision; (c) multicentric disease; and (d) scleroderma or other connective tissue disorders. In all cases, axillary lymphadenopathy or metastatic disease in a sentinel axillary lymph node necessitates an axillary lymph node dissection. In all cases with tumor size >0.5 cm, adjuvant chemotherapy and/or antiestrogen therapy is considered.
Table 1 Definition of TNM | |
|---|---|
|
Locoregionally Advanced Regional Breast Cancer (Stage Iiia or Iiib)
Women presenting with stage IIIa and IIIb breast cancer have advanced locoregional breast cancer but have no clinically detected distant metastases. In an effort to provide optimal locoregional disease-free as well as distant disease-free survival for these women, surgery is integrated with radiation therapy and chemotherapy. Stage IIIa patients are divided into those who have operable disease and those who have inoperable disease (Fig. 1). Surgical therapy for women with operable stage IIIa disease is usually a modified RM, followed by adjuvant chemotherapy, followed by adjuvant radiation therapy. Adjuvant chemotherapy is used to maximize distant disease-free survival, while radiation therapy is used to maximize locoregional disease-free survival. In selected stage IIIa patients, initial (neoadjuvant) chemotherapy is used to reduce the size of the primary cancer and permit conservation surgery. For inoperable stage IIIa and for stage IIIb breast cancer, neoadjuvant chemotherapy is used to decrease the locoregional cancer burden and can permit subsequent surgery to establish locoregional control. In this setting, a modified RM with/without the Patey modification or a Halsted RM is followed by adjuvant chemotherapy and adjuvant radiation therapy.
Table 2 TNM Breast Cancer Stages | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Locoregional Recurrence of Breast Cancer
Local recurrence of breast cancer is best separated into two groups: (a) those having had mastectomy completion and (b) those having had lumpectomy. Patients who had prior mastectomy should undergo surgical resection of the recurrence and appropriate reconstruction. Chemotherapy and/or antiestrogen therapy are considered and adjuvant radiation therapy is given if the chest wall has not previously received breast irradiation. For patients having previous breast conservation, therapy should include mastectomy and appropriate reconstruction. Chemotherapy and/or antiestrogen therapy are considered. Women with recurrent disease involving the axillary lymph nodes (regional recurrence) require axillary lymph node dissection followed by adjuvant chemotherapy.
The more recently utilized mastectomy is the skin-sparing mastectomy, which removes all breast tissue, the nipple–areola complex, and only 1 cm of skin around excised scars. There is a recurrence rate of less than 2% to 4% when skin-sparing mastectomy is used for T1 to T3 cancers. A total
(simple) mastectomy removes all breast tissue, the nipple–areola complex, and necessary skin. An extended simple mastectomy removes all breast tissue, the nipple–areola complex, necessary skin, and the level I axillary lymph nodes. The modified RM removes all breast tissue, the nipple–areola complex, necessary skin, and the level I and II axillary lymph nodes. The Patey modification of the modified RM also removes the pectoralis minor muscle, which permits complete dissection of the apical (level III) axillary lymph nodes. The Halsted RM removes all breast tissue, the nipple–areola complex, necessary skin, the pectoralis major and pectoralis minor muscles, and the level I, II, and III axillary lymph nodes. Currently, chemotherapy, hormone therapy, and radiation therapy for breast cancer have nearly eliminated the need for a Halsted RM.
(simple) mastectomy removes all breast tissue, the nipple–areola complex, and necessary skin. An extended simple mastectomy removes all breast tissue, the nipple–areola complex, necessary skin, and the level I axillary lymph nodes. The modified RM removes all breast tissue, the nipple–areola complex, necessary skin, and the level I and II axillary lymph nodes. The Patey modification of the modified RM also removes the pectoralis minor muscle, which permits complete dissection of the apical (level III) axillary lymph nodes. The Halsted RM removes all breast tissue, the nipple–areola complex, necessary skin, the pectoralis major and pectoralis minor muscles, and the level I, II, and III axillary lymph nodes. Currently, chemotherapy, hormone therapy, and radiation therapy for breast cancer have nearly eliminated the need for a Halsted RM.
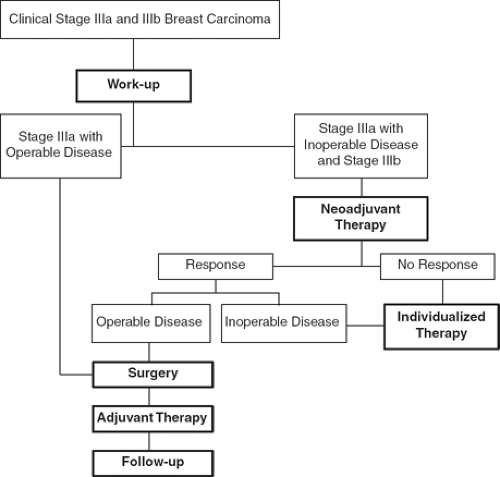 Fig. 1. Treatment pathways for stage IIIa and stage IIIb breast cancer. (Adapted from Bland KI, ed. The Practice of General Surgery. Philadelphia, PA: WB Saunders; 2002, with permission.) |
Positioning: the patient is positioned on the operating table in the supine position for induction of general endotracheal anesthesia (Fig. 2). Rolled towels provide modest nonrestrictive elevation of the ipsilateral hemithorax and shoulder so that shoulder movement is not compromised. Positioning the patient at the edge of the operating table affords the surgeon and the surgical assistant ample access to the breast and axilla and avoids undue retraction on the pectoralis muscle groups or the brachial plexus. The ipsilateral breast, neck, shoulder, and hemithorax are prepped down to the operating table and across the midline of the chest. Folded towels are used to expose the prepped operative field, which includes the shoulder, lower neck, sternum, and upper abdominal musculature. The towels are secured in place with towel clips or surgical staples. In addition, the ipsilateral axilla, arm, and hand are fully prepared within the operative field and the arm is positioned on an arm board that is placed perpendicular to the operating field. While alternative methods exist for including the arm and hand in the operative field, isolation of the hand and forearm with an occlusive cotton dressing (stockinette) is preferred. The stockinette is secured in place by applying an elastic or cotton bandage distal to the ipsilateral elbow, thereby ensuring free mobility of the ipsilateral elbow, arm, and shoulder.
Prior to commencement of the procedure, the first surgical assistant is positioned cephalad to the shoulder of the ipsilateral breast, cephalad to the arm board (Fig. 2). This position permits the assistant to position the arm and shoulder and retract the pectoral muscles appropriately at the time of the axillary dissection. In an obese patient with large breasts, a second surgical assistant can be positioned on the contralateral side of the operating table to assist with exposure of the axilla during axillary dissection.
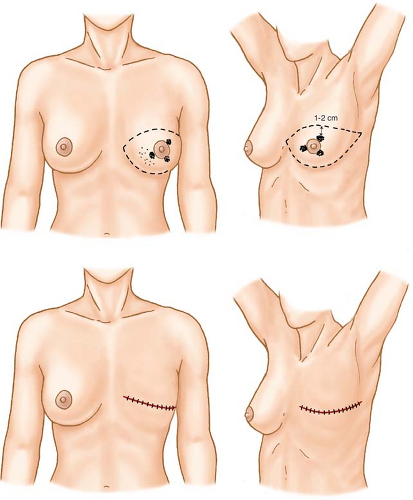
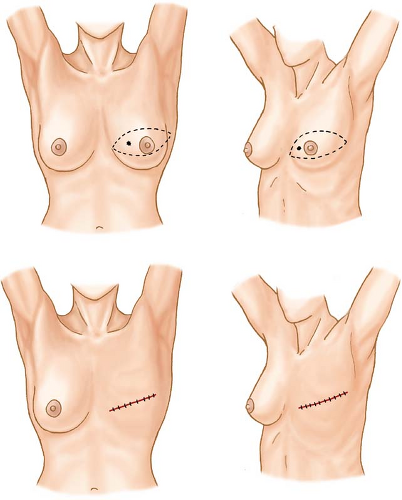
Incisions appropriate for cancers occupying various locations in the breast are shown in Figures 3 to 9. The elliptical incision of the breast skin incorporates the nipple–areolar complex and skin overlying the breast cancer en bloc with skin margins that lie 1 to 2 cm from the cephalad and caudad extents of the cancer.
With electrocautery or scalpel, flaps are developed that extend to the boundaries of dissection for the modified RM and include (a) the anterior margin of the latissimus dorsi muscle laterally; (b) the midline of the sternum medially; (c) the subclavius muscle superiorally; and (d) the caudal extension of the breast, which is 3 to 4 cm inferior to the inframammary fold, inferiorly (Fig. 10, inset). The skin edges are elevated at a right angle to the chest wall to adequately expose the superficial fascia (Fig. 10). Skin flaps include the skin and tela subcutanea and vary in thickness depending on body habitus (Fig. 11A). The appropriate dissection plane for skin flap elevation is deep to the subcutaneous vasculature and superficial to the vessels of the breast parenchyma. The surgeon elevates the skin flap with consistent thickness to avoid creation of devascularized subcutaneous tissues, which can contribute to wound seroma, skin necrosis, and flap retraction (Fig. 11B).
Following the development of skin flaps, the breast parenchyma and pectoralis major fascia is elevated from the underlying pectoralis major muscle in a plane parallel with the muscle bundles as they course from their medial origin (ribs 2 to 6) to their lateral insertion on the humerus (Fig. 11B). Perforating vessels from the lateral thoracic or anterior intercostal arteries, which are end arteries that supply the pectoralis major and minor muscles and breast parenchyma, are regularly encountered during elevation of the breast parenchyma and pectoralis major fascia. These vessels are individually identified and ligated with 2-0 or 3-0 nonabsorbable sutures. Elevation of the breast parenchyma and pectoralis major fascia is continued laterally until the lateral edge of the pectoralis major muscle and the underlying pectoralis minor muscle are exposed. The surgeon is aware of the anatomic location of the lateral neurovascular bundle in which the medial pectoral nerve (laterally placed with origin from the medial cord) courses to innervate the pectoralis
major and minor muscles. If possible, this nerve is preserved to prevent atrophy of the lateral head of the pectoralis major—a significant cosmetic and functional defect (Table 3).
major and minor muscles. If possible, this nerve is preserved to prevent atrophy of the lateral head of the pectoralis major—a significant cosmetic and functional defect (Table 3).
Once elevation of the breast parenchyma and pectoralis major muscle fascia from the underlying pectoralis major muscle is completed, an incontinuity axillary lymph node dissection is performed (Figs. 12, 13). The investing fascia of the axillary space is sharply divided (Fig. 12), the pectoralis minor muscle is defined, and lymph nodes, which may lie between the pectoralis muscles (Rotter’s nodes), are cleared. As the axillary lymph node dissection proceeds, the loose areolar tissue of the lateral axillary space is elevated with identification of the lateral extent of the axillary vein in its course anterior and caudad to the brachial plexus and axillary artery (Fig. 12). (The axillary contents can also be removed in a medial to lateral direction.) The investing layer of the axillary vein is dissected sharply, with dissection allowing complete visualization of the anterior and ventral surfaces of the vein. Ligation and division of intervening venous tributaries is performed. Retraction of the superomedial aspect of the pectoralis major muscle exposes the lateral pectoral nerve, which originates from the lateral cord, and is protected to preserve innervation to the medial heads of the pectoralis major muscle (Figs. 12, 13, Table 3). Dissection continues medially on the anteroventral surface of the axillary vein and the loose areolar tissue at the juncture of the axillary vein with the anterior margin of the latissimus dorsi muscle is swept inferomedially to include the lateral group of axillary lymph nodes (level I, see Fig. 12). The intercostobrachial nerves are infrequently visualized, except for the superior trunk that commonly divides close to the chest wall and courses through the level II axillary lymph nodes that lie below the axillary vein. Generally, no attempt is made to salvage the superior trunk and branches of the intercostobrachial nerve (Table 3).
Preservation of the thoracodorsal artery and vein, located deep in the axillary space, is advocated and these structures are invested with loose areolar tissue and the axillary lymph nodes of the lateral and subscapular groups (Figs. 12, 13). The thoracodorsal nerve originates from the posterior cord medial to the thoracodorsal artery and vein and is visualized and protected along its variable inferolateral course en route to its innervation of the latissimus dorsi muscle. The lateral axillary lymph node group (Fig. 12) is retracted inferomedially and anterior to the thoracodorsal neurovascular bundle and dissected en bloc with the subscapular group of axillary lymph nodes (level I), which are medially located between the thoracodorsal nerve and the lateral chest wall. Dissection of the posterior contents of the axillary space exposes the posterior boundary of the axilla, allowing visualization of the heads of the teres major muscle laterally and the subscapularis muscle medially. Dissection then proceeds medially with extirpation of the central axillary lymph node groups (level II, see Fig. 12, inset). The superomedial aspect of the dissection
specimen can be identified with a metallic marker or suture to provide anatomic orientation for the pathologist. The surgeon continues the dissection en bloc to avoid separation of nodal groups and disruption of lymphatic vessels in the axilla. With medial dissection, the surgeon encounters the chest wall deep in the medial axillary space and is able to identify and preserve the long thoracic nerve (Bell’s respiratory nerve), which is constant in its location, anterior to the subscapularis muscle, and is closely applied to the investing fascial compartment of the chest wall. The long thoracic nerve is dissected from cephad to caudad longitudinal to course and it is completed at point of innervation of serratus anterior muscle (Figs. 12, 14). Damage to the nerve causes permanent disability with a “winged scapula” deformity secondary to denervation of the serratus
anterior muscle (Table 3). The axillary contents anterior and medial to the long thoracic nerve are then swept inferomedially with the dissection specimen. The surgeon ensures that the long thoracic and thoracodorsal nerves are completely visualized before dividing the inferior extent of the axillary dissection.
specimen can be identified with a metallic marker or suture to provide anatomic orientation for the pathologist. The surgeon continues the dissection en bloc to avoid separation of nodal groups and disruption of lymphatic vessels in the axilla. With medial dissection, the surgeon encounters the chest wall deep in the medial axillary space and is able to identify and preserve the long thoracic nerve (Bell’s respiratory nerve), which is constant in its location, anterior to the subscapularis muscle, and is closely applied to the investing fascial compartment of the chest wall. The long thoracic nerve is dissected from cephad to caudad longitudinal to course and it is completed at point of innervation of serratus anterior muscle (Figs. 12, 14). Damage to the nerve causes permanent disability with a “winged scapula” deformity secondary to denervation of the serratus
anterior muscle (Table 3). The axillary contents anterior and medial to the long thoracic nerve are then swept inferomedially with the dissection specimen. The surgeon ensures that the long thoracic and thoracodorsal nerves are completely visualized before dividing the inferior extent of the axillary dissection.
With the presence of level III lymphadenopathy, the Patey modification of the modified RM is employed to enhance locoregional control (Fig. 13). As the surgeon proceeds medially to complete dissection along the lateral margin of the pectoralis major muscle, abduction of the shoulder and extension of the arm along with finger dissection at the lateral margin of the pectoralis major muscle allows visualization
of the insertion of the pectoralis minor muscle on the coracoid process of the scapula. The Patey modification involves division of the tendinous portion of the pectoralis minor muscle (Fig. 12) near its insertion on the coracoid process with/without removal of the muscle, which permits access to the apical axillary lymph nodes (level III) and visualization of the full extent of the axillary vein as it courses beneath the pectoralis minor muscle to its confluence with the subclavian vein beneath the costoclavicular ligament (Halsted’s ligament).
of the insertion of the pectoralis minor muscle on the coracoid process of the scapula. The Patey modification involves division of the tendinous portion of the pectoralis minor muscle (Fig. 12) near its insertion on the coracoid process with/without removal of the muscle, which permits access to the apical axillary lymph nodes (level III) and visualization of the full extent of the axillary vein as it courses beneath the pectoralis minor muscle to its confluence with the subclavian vein beneath the costoclavicular ligament (Halsted’s ligament).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree