CHAPTER 19 Mitochondria, Chloroplasts, Peroxisomes
This chapter considers three organelles formed by posttranslational import of proteins synthesized in the cytoplasm. Mitochondria and chloroplasts both arose from endosymbiotic bacteria, two singular events that occurred about one billion years apart. Both mitochondria and chloroplasts retain remnants of those prokaryotic genomes but depend largely on genes that were transferred to the nucleus of the host eukaryote. Both organelles brought biochemical mechanisms that allow their eukaryotic hosts to acquire and utilize energy more efficiently. In oxidative phosphorylation by mitochondria and photosynthesis by chloroplasts, energy from the breakdown of nutrients or from absorption of photons is used to energize electrons. As these electrons tunnel through transmembrane proteins, energy is partitioned off to create proton gradients. These proton gradients drive the rotary ATP synthase (see Fig. 8-5) to make adenosine triphosphate (ATP), which is used as energy currency to power the cell. Peroxisomes contain no genes and depend entirely on nuclear genes to encode their proteins. Their evolutionary origins are obscure. Peroxisomes contain enzymes that catalyze oxidation reactions that are essential for normal human physiology. Patients who lack peroxisomes have severe neural defects.
Mitochondria
Evolution and Structure of Mitochondria
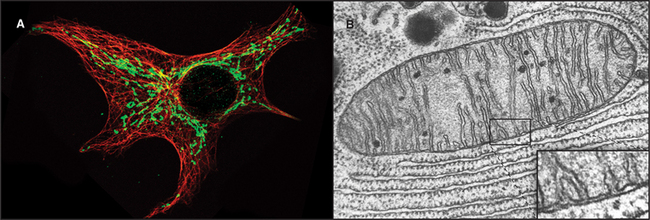
Mitochondria (Fig. 19-1) arose about 2 billion years ago when a Bacterium fused with an archaeal cell or established a symbiotic relationship with a primitive eukaryotic cell (see Fig. 2-5 and associated text). The details are not preserved in the fossil record, but the bacterial origins of mitochondria are apparent in their many common features (Fig. 19-2). The closest extant relatives of the Bacterium that gave rise to mitochondria are Rickettsia, aerobic α-proteobacteria with a genome of 1.1 megabase pairs. These intracellular pathogens cause typhus and Rocky Mountain spotted fever. However, some evidence argues that the actual progenitor bacterium had the genes required for both aerobic and anaerobic metabolism.
As primitive eukaryotes diverged from each other, most of the bacterial genes were lost or moved to the nuclei of the host eukaryotes. The pace of the gene transfer to the nucleus varied considerably depending on the species, but all known mitochondria retain some bacterial genes. A very few eukaryotes, such as Entamoeba, that branched well after their ancestors acquired mitochondria lost the organelle, leaving behind a few mitochondrial genes in the nucleus.
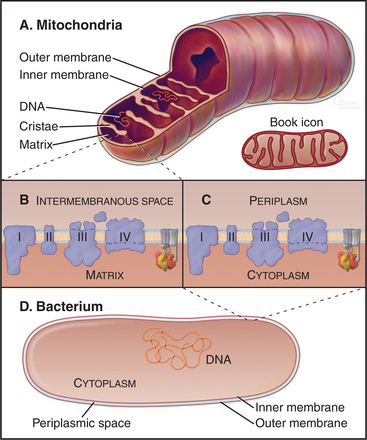
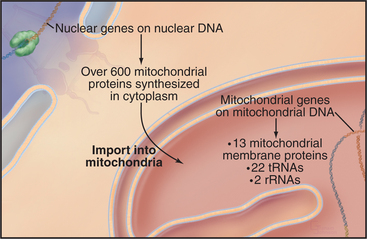
Chromosomes of contemporary mitochondria vary in size from 366,924 base pairs (bp) in the plant Arabidopsis to only 5966bp in Plasmodium. These small, usually circular genomes encode RNAs and proteins that are essential for mitochondrial function, including some subunits of proteins responsible for adenosine triphosphate (ATP) synthesis. The highly pared-down human mitochondrial genome with 16,569bp encodes only 13 mitochondrial membrane proteins, two ribosomal RNAs, and just enough tRNAs (22) to translate these genes. The number of proteins encoded by other mitochondrial genomes ranges from just 3 in Plasmodium to 97 in a protozoan. Nuclear genes encode the other 600 to 1000 mitochondria proteins, including those required to synthesize proteins in the matrix. All mitochondrial proteins that are encoded by nuclear genes are synthesized in the cytoplasm and subsequently imported into mitochondria (see Figs. 18-2 and 18-3).
Mitochondria consist of two membrane-bounded compartments, one inside the other (Fig. 19-2). The outer membrane surrounds the intermembranous space. The inner membrane surrounds the matrix. Each membrane and compartment has a distinct protein composition and functions. Porins in the outer membrane provide channels for passage of molecules of less than 5000D, including most metabolites required for ATP synthesis. The highly impermeable inner membrane is specialized for converting energy provided by breakdown of nutrients in the matrix into ATP. Four complexes (I to IV) of integral membrane proteins use the transport of energetic electrons to create a gradient of protons across the inner membrane. The F1F0 ATP synthase (see Fig. 8-5) utilizes the proton gradient to synthesize ATP. The area of inner membrane available for these reactions is increased by folds called cristae that vary in number and shape depending on the species, tissue, and metabolic state. Cristae may be tubular or flattened sacs. Contacts between the inner and outer membranes are sites of protein import (see Fig. 18-4). Proteins in the intermembranous space participate in ATP synthesis but, when released into the cytoplasm, trigger programmed cell death (see Fig. 46-15).
Biogenesis of Mitochondria
Mitochondria grow by importing most of their proteins from the cytoplasm and by internal synthesis of some proteins and replication of the genome (Fig. 19-3). Targeting and sorting signals built into the mitochondrial proteins that are synthesized in the cytoplasm direct them to their destinations (see Fig. 18-4).
Similar to cells, mitochondria divide, but unlike most cells, they also fuse with other mitochondria. These fusion and division reactions were first observed nearly one hundred years ago. Now it is appreciated that a balance between ongoing fusion and division determines the number of mitochondria within a cell. Both fusion and division depend on proteins with guanosine triphosphatase (GTPase) domains related to dynamin (see Fig. 22-11). In fact, eukaryotes might have acquired their dynamin genes from the bacterium that became mitochondria.
One dynamin-related GTPase is required for division of mitochondria. This GTPase self-assembles into spirals that appear to pinch mitochondria in two. During apoptosis (see Chapter 46), this GTPase also participates in the fragmentation of mitochondria.
Synthesis of ATP by Oxidative Phosphorylation
Mitochondria use energy extracted from the chemical bonds of nutrients to generate a proton gradient across the inner membrane. This proton gradient drives the F1F0 ATP synthase to synthesize ATP from ADP and inorganic phosphate. Enzymes in the inner membrane and matrix cooperate with pumps, carriers, and electron transport proteins in the inner membrane to move electrons, protons, and other energetic intermediates across the impermeable inner membrane. This is a classic chemiosmotic process (see Fig. 11-1).
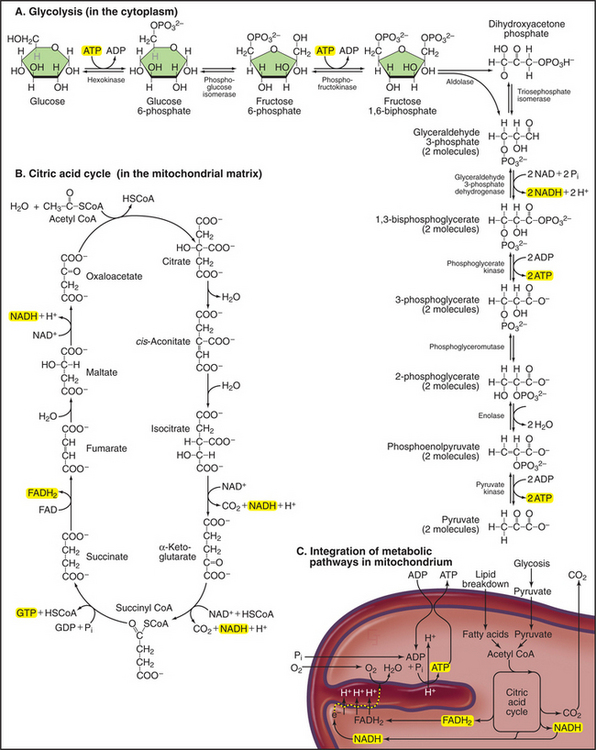
Mitochondria receive energy-yielding chemical intermediates from two ancient metabolic pathways, glycolysis and fatty acid oxidation (Fig. 19-4), that evolved in the common ancestor of living things. Both pathways feed into the equally ancient citric acid cycle of energy-yielding reactions in the mitochondrial matrix:
Breakdown of acetyl-CoA during one turn of the citric acid cycle produces three molecules of NADH, one molecule of FADH2, and two molecules of carbon dioxide. Energetic electrons donated by NADH and FADH2 drive an electron transport pathway in the inner mitochondrial membrane that powers a chemiosmotic cycle to produce ATP (Fig. 19-5). Electrons use two routes to pass through three protein complexes in the inner mitochondrial membrane. Starting with NADH, electrons pass through complex I to complex III to complex IV. Electrons from FADH2 pass through complex II to complex III to complex IV. Along both routes, energy is partitioned off to transfer multiple protons (at least 10 electrons per NADH oxidized) across the inner mitochondrial membrane from the matrix to the inner membrane space. The resulting electrochemical gradient of protons drives ATP synthesis (see Fig. 8-5).
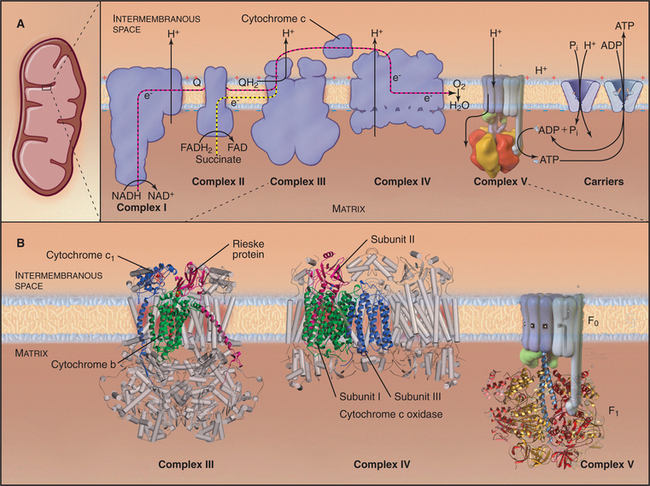
Figure 19-5 chemiosmotic cycle of the respiratory electron transport chain and atp synthase. a, left panel, A mitochondrion for orientation. Right panel, The electron transport system of the inner mitochondrial membrane. Note the pathway of electrons through the four complexes (red and yellow arrows) and the sites of proton translocation between the matrix to the intermembranous space (black arrows). The stoichiometry is not specified, but at the last step, four electrons are required to reduce oxygen to water. ATP synthase uses the electrochemical proton gradient produced by the electron transport reactions to drive ATP synthesis. B, The available atomic structures of the electron transport chain are shown. In the cytochrome bc1 complex III, the 3 of 11 mitochondrial subunits used by bacteria are shown as ribbon models. The supporting subunits found in mitochondria are shown as cylinders. The four subunits of complex IV encoded by the mitochondrial genome are shown as ribbon models. They form the functional core of the complex, which is supported by additional subunits shown as cylinders. See Figure 8-4 for further details of ATP synthase (complex V).
(B, Images of complex III and complex IV courtesy of M. Saraste, European Molecular Biology Laboratory, Heidelberg, Germany. Reference: Zhang Z, Huang L, Schulmeister VM, et al: Electron transfer by domain movement in cytochrome bc1. Nature 392:677–684, 1998. PDB file: 1BCC. Reference: Yoshikawa S, Shinzawα-Itoh K, Nakashima R, et al: Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science 280:1723–1729, 1998. PDB file: 2OCC.)
This process is called oxidative phosphorylation, since molecular oxygen is the sink for energy-bearing electrons at the end of the pathway and since the reactions add phosphate to ADP. Eukaryotes that live in environments with little or no oxygen use other acceptors for these electrons and produce nitrite, nitric oxide, or other reduced products rather than water. Oxidative phosphorylation is understood in remarkable detail, thanks to atomic structures of ATP synthase and three of the four electron transfer complexes. Nuclear genes encode most of the protein subunits of these complexes, but mitochondrial genes are responsible for a few key subunits.
Bacteria and mitochondria share homologous proteins for the key steps in oxidative phosphorylation (Fig. 19-2), but the machinery in mitochondria is usually more complex. Thus, bacteria are useful model systems with which to study the common mechanisms. Plasma membranes of bacteria and inner membranes of mitochondria have equivalent components, and the bacterial cytoplasm corresponds to the mitochondrial matrix (Fig. 19-2).
Energy enters this pathway in the form of electrons that are produced when NADH is oxidized to NAD+, releasing one H+ and two electrons (Fig. 19-5). If the proton and electrons were to combine immediately with oxygen, their energy would be lost as heat. Instead, these high-energy electrons are separated from the protons and then passed along the electron transport pathway before finally recombining to reduce molecular oxygen to form water. Along the pathway, electrons associate transiently with a series of oxidation/reduction acceptors, generally metal ions associated with organic cofactors, such as hemes in cytochromes and iron-sulfur centers (2Fe2S) and copper centers in complex IV. Electrons move along the transport pathway at rates of up to 1000s−1. To travel at this rate through a transmembrane protein complex spanning a 35-nm lipid bilayer, at least three redox cofactors are required in each complex, because the efficiency of quantum mechanical tunneling of electrons between redox cofactors falls off rapidly with distance. Two cofactors, even with optimal orientation, would be too slow.
The first component of the electron transport pathway is called complex I
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree