CHAPTER 31 Intercellular Junctions
The mechanical integrity of animal tissues such as epithelia, nerves, and muscles depends on the ability of the cells to interact with each other and the extracellular matrix. Plasma membrane specializations, called cellular junctions, mediate these interactions. Physical connections from the extracellular matrix or adjacent cells through these junctions and the associated cytoskeletal filaments inside cells impart mechanical strength to tissues.
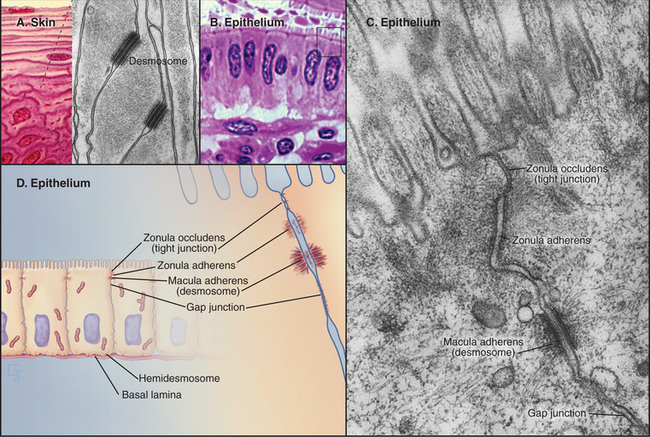
Investigation of junctions began when microscopists and physiologists recognized that epithelial and muscle cells adhere to each other and the underlying extracellular matrix. They also discovered that some epithelia form a tight barrier between the luminal surface and the underlying tissue spaces. The physical basis of these interactions became clear during the 1960s, when electron micrographs of thin sections of vertebrate tissues revealed four types of intercellular junctions that connect the plasma membranes of adjacent cells (Table 31-1 and Fig. 31-1) and two types of junctions to bind to the extracellular matrix. Subsequent research established the molecular architecture of these junctions, each based on a different transmembrane protein:
Vertebrates use a selection of junctions that are suited to the physiological functions of each tissue. Columnar epithelial cells in the intestine interact with their neighbors using all four types of intercellular junctions (Fig. 31-1B-D). Belt-like tight junctions and adherens junctions encircle the apex of the cell. Desmosomes and gap junctions form patch-like lateral connections between the cells. Hemidesmosomes anchor the cells to the basal lamina. Stratified epithelial cells in the skin (Fig. 31-1A) emphasize desmosomes and intermediate filaments (Fig. 31-1B) to resist mechanical forces but also interact via claudins and adherens junctions. Desmosomes and adherens junctions link muscle cells to the surrounding basal lamina (see Fig. 29-18C). Gap junctions connect heart and smooth muscle cells but not skeletal muscle cells. Most nerve cells communicate chemically, but some use gap junctions for electrical communication.
Tight Junctions
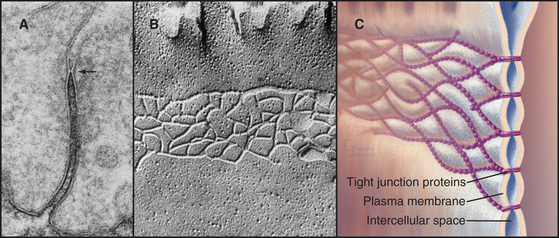
Tight junctions, also called zonula occludens, occlude the extracellular space between epithelial cells, forming a tight, belt-like adhesive seal that selectively limits the diffusion of water, ions, and larger solutes as well as migration of cells (Fig. 31-2). Thus, they separate the interior of the body from the external world. Tight junctions also define the boundary between the biochemically distinct apical and basolateral domains of the plasma membrane of polarized epithelial cells. Many physiological processes (see Figs. 11-2 through 11-4) depend on the selective permeability of the two pathways across an epithelium: passive “paracellular” diffusion through tight junctions and transcellular movement made possible by the action of pumps, carriers, and channels located selectively in the apical and basolateral domains of the plasma membrane.
In electron micrographs of thin sections of tight junctions, the plasma membranes of adjacent cells appear to fuse together in a series of one or more contacts (Fig. 31-2). Early models of tight junctions proposed a fusion between the lipid bilayers of the two membranes to account for the barrier to ion diffusion, but freeze-fracture images revealed that the strands consist of integral membrane proteins. The contacts correspond to continuous strands of intramembranous particles that form a branching network in the plane of the lipid bilayer.
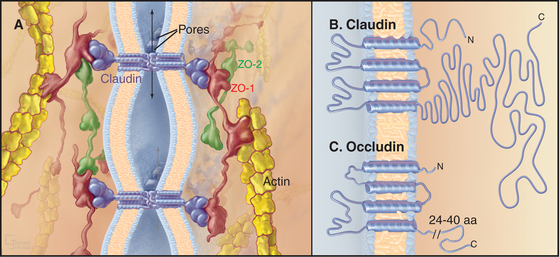
Transmembrane proteins forming the strands ob-served by freeze-fracture were difficult to identify until investigators found a monoclonal antibody that bound to the cytoplasmic side of the plasma membrane at tight junctions. Using this antibody, they isolated an integral membrane protein and named it occludin. The amino acid sequence of occludin suggested four transmembrane strands and two hydrophobic extracellular loops that are extremely rich in tyrosine and glycine residues (Fig. 31-3). The same group then discovered that mice lacking the single occludin gene survive with normal tight junctions, revealing the existence of other tight junction proteins. It is now believed that a family of more than 20 proteins, called claudins, constitutes the main structural proteins of tight junction strands. Claudins have four transmembrane sequences, but they are not related in sequence to occludin. Close association of claudins limits diffusion in the extracellular space between cells as well as diffusion of lipids and proteins in the plane of the membrane.
The cytoplasmic tails of claudins interact with numerous proteins with roles as scaffolds and in actin binding, signaling, and cell polarity. ZO-1, ZO-2, and ZO-3, peripheral membrane adapter proteins containing PDZ protein-interaction domains (see Fig. 25-11), interact with the long C-terminal cytoplasmic tail of occludin and JAM (junctional adhesion molecule). These ZO-adapters link the transmembrane proteins to cytoplasmic proteins including actin filaments, cingulin, and a small GTPase. ZO-2 and cingulin are specific for tight junctions, whereas ZO-1 also associates with cadherins in adherens junctions and connexins in gap junctions.
Tight junctions are the barrier that segregates different pumps, carriers, receptors, and lipids in the apical and basolateral domains of the plasma membrane. These domains allow polarized cells to create different extracellular environments in the basal and apical compartments. For example, intestinal epithelial cells take up nutrients from the lumen of the intestine and transport them into the extracellular space beneath the cells (see Fig. 11-2). Tight junctions and epithelial polarity are required for many physiological functions (see Figs. 11-3 and 11-4). Separating the apical and basal compartments also regulates some types of signaling. For instance, airway epithelial cells release the growth factor heregulin from the apical surface, but it activates cell growth only by activating the receptor tyrosine kinase erbB2 (see Fig. 25-4) on the basolateral surface when the epithelium is damaged or the tight junctions compromised.
The transepithelial barrier that is established by tight junctions is regulated by extracellular stimuli (e.g., hormones such as vasopressin and aldosterone and cytokines such as tumor necrosis factor; see Chapter 27), their downstream second messengers (e.g., Ca2+ and cyclic adenosine monophosphate [cAMP]; see Chapter 26), and effectors (e.g., protein kinases A and C; see Chapter 25). The mechanisms are not yet well understood, but posttranslational modifications of tight junctions might modulate their assembly. Another possibility is that tension on associated actin filaments might physically open passages through tight junctions. The metabolic state of the cell also influences tight junctions; depletion of ATP causes tight junctions to leak without destroying the barrier between the apical and basolateral domains of the plasma membrane. Cells migrating across epithelia, such as white blood cells moving from the blood to the connective tissue, open tight junctions locally without disrupting the tight seal across the epithelium (see Fig. 30-13). Migratory cells induce a localized increase in cytoplasmic Ca2+ in the epithelial cells that is required for opening the tight junctions.
Gap Junctions
For many years, the dominance of the cell theory in biology, which suggested that isolation of cells was a general principle, discouraged curiosity about the possibility of direct intercellular communication. Early electrophysiological experiments on nerves and skeletal muscles reinforced the widespread belief that cells were autonomous. By chance, the cells that were used in these experiments were electrically isolated and communicated exclusively by secreting chemical messengers that bound to receptors on target cells (see Figs 11-8 and 11-9). However, nerve and skeletal muscle cells were found to be exceptions to the general principle, which emerged only later, that cells in animal tissues communicate with each other by gap junctions. The generality of gap junctional communication means that sharing cytoplasmic components is common in animal cell biology. Cells in plant tissues also communicate with each other, but they use direct cytoplasmic connections, called plasmodesmata, rather than gap junctions (Box 31-1 and Fig. 31-4).