Minimally Invasive Parathyroidectomy
Robert Udelsman
Tobias Carling
Background
Approximately 85% of patients with primary hyperparathyroidism (pHPT) harbor a single adenoma and are cured by excision of the incident gland. The remaining patients display double adenomas (3% to 5%) or four-gland hyperplasia (10% to 15%). Thus, with accurate preoperative localization, targeted surgery using unilateral neck exploration under regional or local anesthesia has been developed; moreover, it has been evaluated over the past decade and has become the standard of care in an ever-increasing number of specialized centers. Unilateral surgery for pHPT was advocated in 1975, and the side to be explored was chosen based on palpation, esophageal imaging, venography, or arteriography. If both an enlarged and normal gland were found on the initial side, then contralateral cervical exploration was obviated. Other authors advocated a similar approach, arguing that bilateral exploration increased the risk, cost, and morbidity of surgery for pHPT. The Lund University surgeons advocated unilateral parathyroidectomy, which they defined as removal of both an adenoma and ipsilateral normal parathyroid gland. The excised tissue was studied microscopically during surgery with oil-red-O, and the decision to stop the operation at this stage was based on demonstration of a reduction in intracytoplasmic fat droplets in the excised adenomatous parathyroid tissue. Both techniques would fail, however, in the setting of a double adenoma on the contralateral side and if the essentially “random” choice of which side to explore was incorrect. Today, minimally invasive parathyroidectomy (MIP) is performed after preoperative parathyroid localization usually with high-quality sestamibi scans, ultrasonography, or four-dimensional parathyroid computed tomography (CT) scans, often under cervical block anesthesia during which a focused exploration is performed, and the rapid intraoperative parathyroid hormone assay is employed to confirm an adequate resection.
The indications for MIP are the same as those for traditional cervical exploration, that is, symptomatic patients or those with asymptomatic pHPT fulfilling the criteria established by the most recent National
Institutes of Health (NIH) consensus meeting. In addition, there are now significant data to support more liberal use of parathyroidectomy for pHPT, since the disease has been associated with a number of “nonclassical” morbidities, some of which seem to improve postoperatively. These include neurocognitive impairments and cardiovascular abnormalities. Patients with persistent disease (previously failed parathyroidectomy) as well as other reoperative cases can also be successfully operated on using MIP techniques. Minimally invasive techniques are rarely employed when preoperative localization of the parathyroid tumor was not performed, is negative, or is consistent with multiglandular enlargement. The role of MIP in the setting of familial hyperparathyroidism (HPT), that is, multiple endocrine neoplasia type 1 (MEN1), MEN2A, the hyperparathyroidism–jaw tumor syndrome (HPT-JT), familial isolated hyperparathyroidism (FIHPT), and HPT occurring in patients with an underlying mutation in the calcium sensing receptor (CASR) gene, is evolving. A conventional cervical exploration should be performed in the vast majority of these cases, although MIP may prove to have a limited role in specific instances of familial HPT. For instance, in HPT associated with MEN2A, HPT-JT, and FIHPT where uniglandular uptake is noted on preoperative imaging, MIP may be considered. Rare cases when parathyroid carcinoma is diagnosed or suspected, a radical resection at the initial operation is required for optimal results.
Institutes of Health (NIH) consensus meeting. In addition, there are now significant data to support more liberal use of parathyroidectomy for pHPT, since the disease has been associated with a number of “nonclassical” morbidities, some of which seem to improve postoperatively. These include neurocognitive impairments and cardiovascular abnormalities. Patients with persistent disease (previously failed parathyroidectomy) as well as other reoperative cases can also be successfully operated on using MIP techniques. Minimally invasive techniques are rarely employed when preoperative localization of the parathyroid tumor was not performed, is negative, or is consistent with multiglandular enlargement. The role of MIP in the setting of familial hyperparathyroidism (HPT), that is, multiple endocrine neoplasia type 1 (MEN1), MEN2A, the hyperparathyroidism–jaw tumor syndrome (HPT-JT), familial isolated hyperparathyroidism (FIHPT), and HPT occurring in patients with an underlying mutation in the calcium sensing receptor (CASR) gene, is evolving. A conventional cervical exploration should be performed in the vast majority of these cases, although MIP may prove to have a limited role in specific instances of familial HPT. For instance, in HPT associated with MEN2A, HPT-JT, and FIHPT where uniglandular uptake is noted on preoperative imaging, MIP may be considered. Rare cases when parathyroid carcinoma is diagnosed or suspected, a radical resection at the initial operation is required for optimal results.
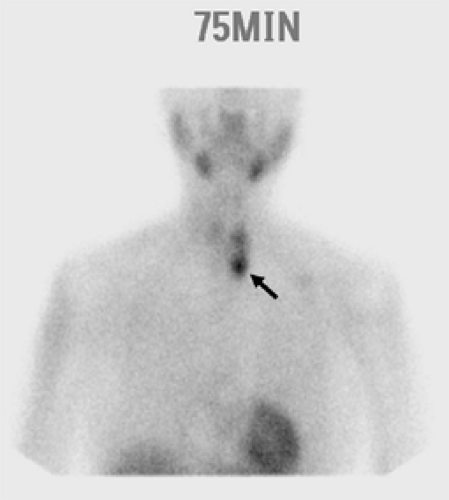
The development and refinement of parathyroid imaging has been essential for the development of MIP techniques. Several noninvasive preoperative localization methods are available, including sestamibi–technetium-99m scintigraphy, ultrasonography, CT, magnetic resonance imaging (MRI), and thallium-201–technetium-99m pertechnetate scanning. The most commonly used modality remains sestamibi with single-photon emission computed tomography (SPECT), which generates three-dimensional localization (Fig. 1). In 1989, it was first reported that the new agent technetium-99m used for cardiac imaging was also avidly taken up by parathyroid tissue. Parathyroid cells have a large number of mitochondria, which take up sestamibi/technetium-99m. Sestamibi, a monovalent lipophilic cation, diffuses passively across cell membranes, concentrates in mitochondria, and accumulates in adenomatous parathyroid tissue because of increased blood supply, higher metabolic activity, and an absence of p-glycoprotein on the cell membrane. Sestamibi imaging can be performed preoperatively to plan a MIP or on the morning of operation in combination with the use of a gamma probe in the operating room to guide the surgeon during the operation. A meta-analysis of the sensitivity and specificity of sestamibi scanning in 6,331 cases demonstrated values of 90.7% and 98.8%, respectively, and suggested that 87% of the patients with sporadic pHPT would be candidates for a unilateral exploration. The sensitivity of sestamibi is limited in multiglandular disease. In a large study, scintigraphy localized at least one gland in all patients, but only 62% of the total number of hyperplastic glands. SPECT, which allows localization of structures in the anterior/posterior plane, is particularly helpful in detecting smaller lesions and adenomas located posterior to the thyroid gland. The overall sensitivity for localizing adenomas smaller than 500 mg varies, from 53% to 92%. A major limitation of sestamibi scans is related to the coexistence of thyroid nodules or other metabolically active tissues (e.g., lymph nodes, thyroid nodules, and metastatic thyroid cancer) that can mimic parathyroid adenomas, thereby causing false-positive results on sestamibi scans. This limitation can be overcome in part by using the double-tracer subtraction technique of sestamibi, in which both thyroid and parathyroid nodular abnormalities can be diagnosed simultaneously, or in combination with a neck ultrasound to preoperatively distinguish between thyroid lesions and parathyroid enlargement. Sestamibi with SPECT does not provide detailed anatomical depiction, and can only detect double adenomas and multiglandular hyperplasia in 25% to 45% of cases.
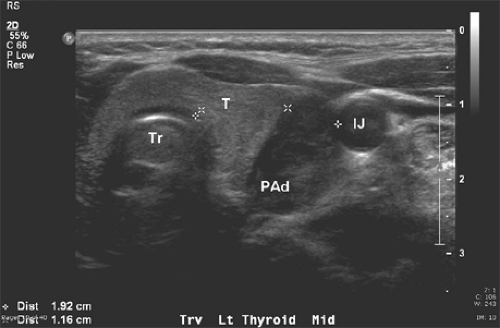 Fig. 2. Corresponding cervical ultrasound from the same patient as in Figure 1, showing a large hypoechoic cystic parathyroid adenoma (PAd). The left thyroid (T), trachea (Tr), and left internal jugular vein (IJ) are indicated. |
Ultrasound is effective, noninvasive, and inexpensive, but its limitations include both operator dependency and its application being limited to the neck because it cannot image mediastinal adenomas. The normal parathyroid gland is generally too small to be visualized sonographically, whereas the parathyroid enlargement seen in pHPT is often identified as a homogeneously hypoechoic extrathyroidal ovoid mass (Fig. 2). Parathyroid adenomas are typically vascular and an arterial branch can often be followed to the superior or inferior pole of the lesion. An additional advantage of ultrasound is the ability to perform fine needle aspirates with rapid parathormone (PTH) measurements of the aspirates, especially in patients undergoing reexploration. By itself, ultrasound has approximately a 50% to 75% true-positive rate, with generally better rates for larger glands. However, when combined with sestamibi, the true-positive rate
approaches 90%, with few false-positives. The ability of both ultrasound and sestamibi with SPECT to detect parathyroid abnormality is reduced in milder forms of pHPT (i.e., only mildly elevated PTH and calcium levels) and in obese patients.
approaches 90%, with few false-positives. The ability of both ultrasound and sestamibi with SPECT to detect parathyroid abnormality is reduced in milder forms of pHPT (i.e., only mildly elevated PTH and calcium levels) and in obese patients.
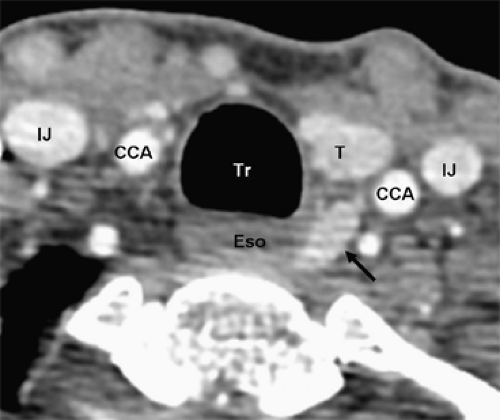
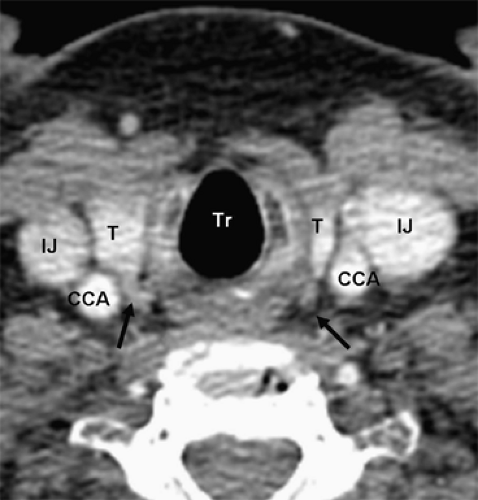
Four-dimensional parathyroid CT scan (4DCT) is a promising new parathyroid imaging technique. Also, 4DCT is similar to CT angiography. The term is derived from three-dimensional CT scanning with an added dimension referring to the changes in perfusion of contrast over time. Exquisitely detailed, multiplanar images are obtained that accentuate the differences in the perfusion characteristics of hyperfunctioning parathyroid glands (i.e., rapid uptake and washout), compared with normal parathyroid glands and other structures in the neck (Figs. 3 and 4). The images provide both anatomic information and functional information (based on changes in perfusion) in a single study that the operating surgeon can easily interpret. In a recent series, 4DCT displayed improved sensitivity (88%) over sestamibi imaging (65%) and ultrasonography (57%), when the imaging studies were used to localize (lateralize) hyperfunctioning parathyroid glands to one side of the neck. Moreover, when used to localize parathyroid tumors to the correct quadrant of the neck (i.e., right inferior, right superior, left inferior, or left superior), the sensitivity of 4DCT (70%) was significantly higher than sestamibi imaging (33%) and ultrasonography (29%). In a prospective consecutive cohort, the results at Yale University are similar. Moreover, 4DCT predicted multiglandular disease (Fig. 4) in 85.7% (6/7) patients. Compared with sestamibi with SPECT, 4DCT is significantly less expensive but associated with higher exposure to ionizing radiation and thus should be used cautiously in children and young adults. Moreover, due to the use of intravenous contrast it should be avoided in patients with renal insufficiency as well as in patients with concomitant well-differentiated thyroid carcinoma. With the development of improved CT scanning, MRI is rarely used. However, since it does not involve the use of radiation it may be employed in select cases. Parathyroid adenomas may appear very intense on T2-weighted images.
A subset of patients who require reexploration will have negative, discordant, or nonconvincing, noninvasive localization studies. Current guidelines recommend that these patients undergo invasive localization procedures in the form of selective venous sampling (SVS) with measurements of PTH. Rapid PTH measurement is now being used in the angiography suite, because results are rapidly available on site, and interventional radiologists can obtain additional samples from a region in which a subtle but potentially significant PTH gradient is detected. In a recent study, SVS had a sensitivity of 83.3% for the correct localization of a parathyroid adenoma or hyperplastic parathyroid glands, whereas false-positive or indeterminate results of SVS were found in 6% and 2% of cases, respectively.
Regional Block Anesthesia Technique
The majority of parathyroidectomies are performed under general anesthesia using either an endotracheal tube (ETT) or laryngeal mask airway (LMA). We prefer local and regional block anesthesia with monitored anesthesia care (MAC). The regional block is performed by the surgeon in the
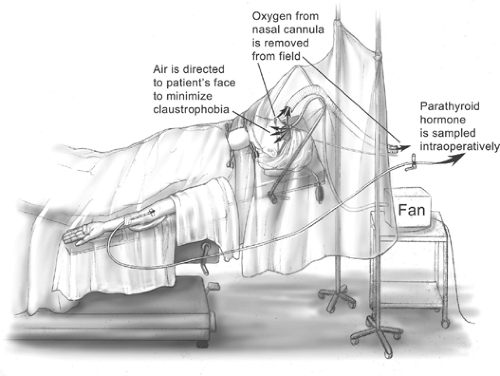
operating room, and intravenous supplementation is administered by the anesthesia staff (Fig. 5). In most patients, 1% lidocaine containing 1:100,000 epinephrine is used and supplemented during the operation as required. Care is taken to aspirate before delivering the anesthetic to avoid intravascular administration. We have found that by also infiltrating along the anterior border of the sternocleidomastoid muscle, as well as a local field block, excellent analgesia is obtained in virtually every case (Fig. 6). The total cumulative volume of lidocaine administered is typically 18 to 25 mL. Intravenous sedation is used to minimize patient anxiety while maintaining an awake, conscious patient who can phonate. Propofol was thought to possibly interfere with the PTH assay, but a recent randomized trial has shown that the PTH assay can be employed during propofol sedation.
operating room, and intravenous supplementation is administered by the anesthesia staff (Fig. 5). In most patients, 1% lidocaine containing 1:100,000 epinephrine is used and supplemented during the operation as required. Care is taken to aspirate before delivering the anesthetic to avoid intravascular administration. We have found that by also infiltrating along the anterior border of the sternocleidomastoid muscle, as well as a local field block, excellent analgesia is obtained in virtually every case (Fig. 6). The total cumulative volume of lidocaine administered is typically 18 to 25 mL. Intravenous sedation is used to minimize patient anxiety while maintaining an awake, conscious patient who can phonate. Propofol was thought to possibly interfere with the PTH assay, but a recent randomized trial has shown that the PTH assay can be employed during propofol sedation.
Regional anesthesia avoids complications associated with general anesthesia, including endotracheal intubation, which has been reported to cause vocal cord changes in up to 5% of patients. Furthermore, exploring a conscious patient permits intraoperative assessment of the superior and recurrent laryngeal nerve functions because the patient can vocalize during the procedure.
LoGerfo and colleagues have shown that bilateral neck exploration under regional anesthesia can be performed safely and effectively in patients with coexisting thyroid disease and a nonlocalized adenoma. In a series of 236 patients undergoing MIP, 62% had a nonlocalizing sestamibi scan preoperatively or no scan at all, but only four required conversion to general anesthesia. Twenty-three percent had a simultaneous procedure performed for thyroid disease, and 85% underwent a bilateral neck exploration. We recently reported in 441 consecutive patients that 47 (10.6%) required conversion to general anesthesia. In all instances, conversion was performed in a controlled fashion using neuromuscular blockade, endotracheal intubation, and maintenance of the original surgical field preparation. Table 1 summarized the reasons for conversion from regional block to general endotracheal anesthesia.
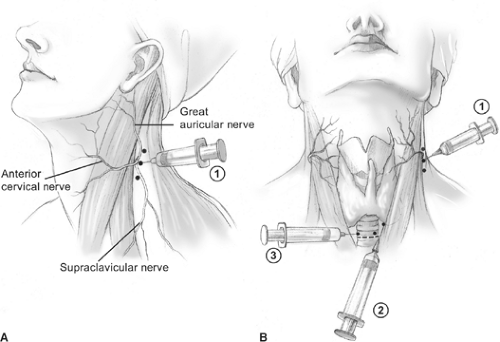 Fig. 6. Cervical block anesthesia. A: A superficial cervical block is administered posterior and deep to the sternocleidomastoid muscle (SCM) (1). B: Local infiltration is also performed along the anterior border of the SCM (2), and a local field block (3) is performed.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|