Minimally Invasive Left Hepatic Lobectomy
Trang K. Nguyen
Amer H. Zureikat
DEFINITION
A minimally invasive left hepatic lobectomy (also termed left hepatectomy or left hemihepatectomy) is the resection of the liver medial to the midplane of the liver (Cantlie’s line) using laparoscopic or robotic assistance and is formally defined as the resection of Couinaud segments 2, 3, and 4.1
A left trisectionectomy or extended left hepatectomy (or hemihepatectomy) would also include the adjacent segments 5 and 8 with or without segment 1. A left lateral sectionectomy involves only segments 2 and 3.
A liver resection is termed laparoscopic if it is a pure laparoscopic procedure, a hand-assisted procedure, or a hybrid technique procedure (which begins as a pure laparoscopic or as a hand-assisted procedure with the liver resection done through a minilaparotomy incision).2
A left hepatectomy is performed for benign and malignant lesions and for living donor transplantation.
PATIENT HISTORY AND PHYSICAL FINDINGS
A comprehensive history and physical exam should be performed with attention to signs and symptoms of liver failure, coagulopathy, and cardiac disease.
The same indications for an open left hepatectomy apply to a minimally invasive left hepatectomy and the indications for benign lesions should not be relaxed due to the minimally invasive approach.2 The indications for resection include hepatic adenoma, symptomatic hemangiomas, symptomatic focal nodular hyperplasia, symptomatic giant cysts, hepatocellular carcinoma, and colorectal cancer metastases.3
The contraindications for a minimally invasive left hepatectomy include those for an open resection, in addition to decompensated cirrhosis, the inability to tolerate pneumoperitoneum, dense adhesions that are not amenable to minimally invasive adhesiolysis, need for extensive portal lymphadenectomy, need for vascular resection, and lesions that are near major vessels.3,4 Biliary reconstruction is a relative contraindication for robotic-assisted surgery depending on the experience of the surgeon.5
Lesion characteristics that are most favorable for laparoscopic resection include solitary lesions, size of 5 cm or less, peripheral location, and a lack of involvement of the hilum, major hepatic veins, or the inferior vena cava (IVC).2
IMAGING AND OTHER DIAGNOSTIC STUDIES
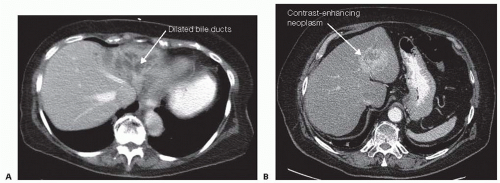
Preoperative imaging is required to evaluate lesion resectability and for surgical planning. Imaging is useful for evaluation of lesion size, proximity to major vessels and bile ducts, aberrant anatomy, adequacy of anticipated postoperative liver volume, and for detection of lung or other abdominal metastases for malignant indications.
Contrast-enhanced computed tomography (CT) (FIG 1), magnetic resonance imaging (MRI), and positron emission tomography (PET) can be used for preoperative evaluation.6 A triple-phase CT (arterial, venous, and delayed venous phase) is useful for its spatial resolution and volumetric assessment. MRI is superior for detecting subcentimeter lesions. PET scans may be useful for detecting other sites of metastasis.
If a quantitative evaluation of liver function is needed, especially for patients who have undergone hepatotoxic chemotherapy, a monoethylglycinexylidide (MEGX) test or indocyanine green clearance test can be used.6
Occasionally, preoperative liver biopsy may be necessary to document the presence or extent of cirrhosis.
Preoperative laboratory tests include a complete blood count, liver function tests, coagulation profile, and relevant tumor markers in cases of malignant disease.
SURGICAL MANAGEMENT
Preoperative Planning
Minimally invasive hepatic surgery should be performed by surgical teams experienced in both advanced minimally invasive techniques and major hepatic surgery.
All potentially necessary equipment should be readily available and the patient should be counseled on the possibility for conversion to an open procedure.
Preoperative portal vein embolization (PVE) can be considered in order to increase the size of the future liver remnant (FLR) to a minimum of 25% in the absence of cirrhosis and a minimum of 40% to 50% in well-compensated cirrhosis. PVE is less commonly needed in a left hepatectomy as compared to a right hepatectomy.7
Positioning
Arrange the operating room so that the robot can be docked at the head of the table.
Place the patient in supine position on a split-leg table in slight reverse Trendelenburg position. Video monitors are placed to the right and left of the head of the patient.
Insert a central venous and arterial lines for hemodynamic monitoring. Give preoperative antibiotic prophylaxis and have crossmatched blood available.
Judiciously administer intravenous fluids; keep the central venous pressure (CVP) less than 5 cm H2O.
For a purely laparoscopic resection (no robotic assistance), the operation can be performed with the surgeon standing between the patient’s legs or with the surgeon at the patient’s left during the hilar dissection and then moving to the right side for the parenchymal transection.8
For a robotic resection:
Laparoscopic portion (liver mobilization): The surgeon stands on the left side, the assistant on the right, and the camera assistant between the legs.
Robotic portion (control of vascular inflow, parenchymal transection, hepatic outflow): The surgeon sits at the console and the assistant is between the legs (facilitates retraction, suction/irrigation, bipolar electrocautery, suture exchange, exchange of robotic instruments, and endovascular stapling).
TECHNIQUES
PORT PLACEMENT
Establish pneumoperitoneum via a 5-mm optical separator port in the left mid-abdomen approximately one handbreadth to the left and 2 in above the umbilicus.
After pneumoperitoneum is established, place a 10-mm camera port in the right mid-abdomen approximately one handbreadth to the left and 2 in above the umbilicus. This position is a mirror image of the previous port.
The abdomen is explored for metastatic disease.
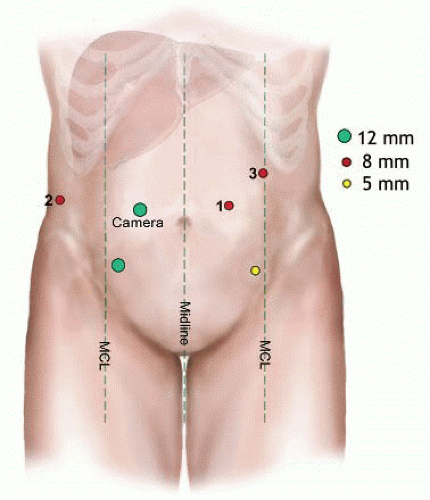
The full complement of ports is then placed as shown in FIG 2:
Three 8-mm robotic ports—R2 on the right and R1 (initial port upsized to robotic) and R3 on the left
One 12-mm camera port
One 12-mm laparoscopic assistant port in right lower quadrant (for stapling, suture access, energy device, suction, and clip applier)
One 5-mm laparoscopic assistant port in left lower quadrant cases (for energy device, suction, and clip applier)
For hepatomegaly or with large tumors, the robotic ports are moved inferiorly toward the level of the umbilicus.8
An intraabdominal pressure of less than 12 mmHg is recommended. Although a high intraabdominal pressure can theoretically improve hemostasis during liver transection, a high intraabdominal pressure could also potentially increase the risk of gas embolism, particularly if injury to the hepatic veins is encountered.9
EXPLORATION AND LESION EVALUATION
The abdominal cavity and the liver surface are again examined for metastases. All suspicious lesions should be sent for frozen section biopsy.
Laparoscopic ultrasound of the liver is then performed to confirm resectability of the targeted lesion(s) and the proximity to major vessels as well as to rule out other hepatic metastases.
LIVER EXPOSURE AND MOBILIZATION
This portion of the operation is performed laparoscopically.
Divide the falciform ligament and the ligamentum teres with hook electrocautery/bipolar electrocautery. Alternatively, this step can be performed prior to the intraoperative ultrasound. The ligamentum teres is left long enough to be used as a grasping point during the portal dissection.
Divide the left triangular and left coronary ligaments using hook electrocautery or another energy device. (FIG 3A,B)
Open the pars flaccida, staying close to the undersurface of the left lateral section and the caudate lobe. An accessory left hepatic artery may be encountered and is doubly clipped and divided.
Incise Arantius’ ligament using the hook cautery. It runs from the left branch of the portal vein to the left hepatic vein or the common (left and middle) hepatic vein truck. This maneuver must be performed with great care because injury to the left hepatic vein can occur. Alternatively, it can be done after the robot is docked to improve visualization.
The bridge between the left lateral and medial sections (segments 3 and 4B) is divided.
A cholecystectomy is performed to facilitate with exposure of the porta hepatis.10
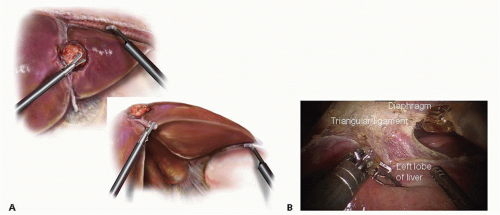 FIG 3 Divide the left triangular ligament using a laparoscopic or robotic hook cautery. Start the dissection lateral and work medial until the left hepatic vein is visualized as it enters the IVC. Using the falciform ligament as a handle (inset) for exposure, incise the pars flaccida to expose the ligament of Arantius. Dissect this ligament up to the left hepatic vein. B. Intraoperative view of the mobilization of the left lobe of the liver.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|