[Note: This equation is the shortcut of the following:
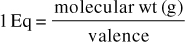
Then, this last equation is converted to milliequivalents (mEq) by dividing both sides by 1000, which yields the equation above. As a shortcut, then, the equation can be used remembering that in this instance, the unit of milligram is affixed to the molecular weight.]
Of note is that the molecular or atomic weights are usually assigned the unit of gram; however, for milliequivalents we assign the unit of milligram. The weight is not converted from gram to milligram. For example, if the molecular weight was given as 180 for a divalent electrolyte, the value of 180 mg/2 would be the conversion factor.
Once the amount of drug is converted to its metric equivalent, the concentration can be converted to any other unit of concentration, such as percent, ratio strength, or weight per unit volume.
Also, determining the amount, in milliequivalents, of the parent compound (eg, potassium chloride), automatically determines the amount, in milliequivalents, of the individual electrolytes (ie, for potassium ion and chloride ion). One milliequivalent of the parent compound will generate one milliequivalent of the cation (positively charged) electrolyte and one milliequivalent of the anion (negatively charged) electrolyte.
Practice Exercises
Calculate the amount of electrolyte in each problem.
1. The patient is to take 40 mEq of potassium in the form of potassium chloride solution. How many milligrams of potassium does this represent? MW = 75
Answer
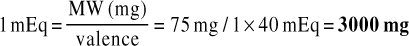
2. How much calcium chloride is required to make 500 mL of solution such that the final concentration is 4 mEq/mL? MW = 147
< div class='tao-gold-member'>



