and Natalia Buza1
(1)
Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
Keywords
Metastatic tumors of ovaryMetastatic intestinal adenocarcinomaKrukenberg tumorMetastatic pancreatobiliary adenocarcinomasMetastatic appendiceal tumorsMetastatic breast carcinomaMetastatic cervical adenocarcinoma, usual typeIntroduction
Metastatic tumors account for up to 15 % of ovarian malignancies in western countries [1, 2] and may present a significant diagnostic challenge during intraoperative frozen section consultation. The primary site is most commonly located in the gastrointestinal tract, with approximately 30 % of cases originating from the colon, followed by appendiceal, gastric, and pancreatobiliary primaries [3, 4]. Among non-gastrointestinal primaries, breast cancer is the most frequent source of ovarian metastases [3, 5], which may also be an incidental finding in prophylactic or therapeutic oophorectomy specimens. Metastatic endometrial and cervical carcinomas to the ovary occur at a lower frequency and generally have less severe implications on the intraoperative management at the time of frozen section compared to other metastatic sites. While comprehensive surgical staging is typically performed for primary ovarian malignancies, gynecologic staging surgery is unnecessary for extragenital primary tumors metastatic to the ovary. Intraoperative frozen section evaluation plays a critical role in this distinction and therefore guides the extent of surgery.
The most important first step in assessing the origin of tumor—primary versus metastatic—is thorough review of the patient’s clinical history. However, the primary tumor may be occult and the metastasis may precede the diagnosis of primary tumor. In one study, 51 % of ovarian metastases of gastrointestinal origin were diagnosed prior to the discovery of the primary tumor [3]. The median age of patients with ovarian metastases is 53–55 years [4, 5], although patients with Krukenberg tumor typically present earlier (average age: 45 years) [6]. The clinical presentation may be similar to primary ovarian tumors—pelvic pain and mass effect, and rarely even hormonal (androgenic or estrogenic) manifestations, due to functioning stroma [6]. The macroscopic features may be helpful, as bilaterality, tumor size under 10 cm, ovarian surface involvement, and multinodular growth pattern are more common in metastatic tumors [7]; however, exceptions do occur. On microscopic examination, extensive lymphovascular invasion and ovarian hilus involvement are generally in favor of metastatic carcinoma. Comparison with the patient’s known primary should be made if the prior specimen is available at the time of frozen section, although the histological features at the metastatic site may slightly be different from those of the primary tumor. For example, mucinous tumors metastatic to the ovary may demonstrate a “maturation phenomenon,” mimicking a primary benign or borderline ovarian mucinous tumor [8]. Cystic growth pattern may be encountered in the ovarian metastasis, even when the primary tumor is predominantly solid [6].
The frozen section diagnosis—especially in difficult or equivocal cases—should not be made in isolation without intraoperative discussion with the surgeon. If only one ovary is received for frozen section, the surgeon should be inquired about the appearance of the other ovary and about the possibility of bilaterality, especially if dealing with a mucinous tumor. If a metastasis is suspected, intraoperative surgical evaluation of the pelvis and abdomen and sampling from any additional pelvic or intra-abdominal—intestinal, appendiceal, or gastric—lesions or enlarged intra-abdominal lymph nodes may be helpful. Intraoperative identification of pseudomyxoma peritonei by the surgeon or presence of extracellular mucinous material on the ovarian surfaces strongly favors appendiceal primary. Appendectomy is usually performed in this scenario even if the appendix appears grossly unremarkable.
Metastases to other gynecologic organs occur less commonly and may involve vagina, vulva, uterine cervix, endometrium, and fallopian tube—in decreasing order of frequency [5]. Metastatic tumors may mimic primaries at these sites and may be easily misinterpreted, especially due to their rare occurrence. Unusual morphologic features—e.g., intestinal-type differentiation with goblet cells or extensive lymphovascular invasion—should raise suspicion for a possible metastatic origin.
This chapter summarizes the clinical, gross, and microscopic features and site-specific key intraoperative consultation issues of the most common metastatic tumors—intestinal, signet-ring cell, pancreatobiliary and appendiceal adenocarcinomas, and breast and gynecologic primaries—involving the ovary. Metastases from other primary sites—lung, urinary tract, melanoma, and sarcomas—occur rarely, and their clinical and histopathologic features depend largely on the primary tumor site.
Gastrointestinal Tract
Intestinal Adenocarcinoma
Clinical and gross features
Most frequent tumor type among ovarian metastases.
May have functioning ovarian stroma.
Most common primary site is rectosigmoid (77 %), followed by cecum (9 %), ascending colon (9 %), and descending colon (5 %) [9].
Often bilateral, with a solid, or solid and cystic tan-yellow, or gray cut surface (Fig. 12.1).
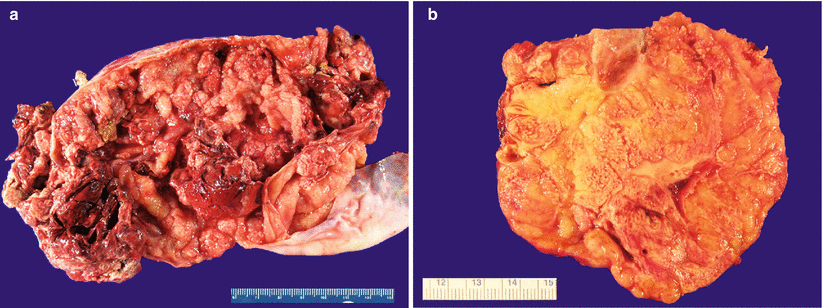
Fig. 12.1
Metastatic colorectal adenocarcinoma. Note the predominantly cystic (a) or solid, tan-pink, and multinodular cut surface (b)
Ovarian surface involvement and multinodular growth pattern are commonly seen.
Necrosis and hemorrhage are common.
Microscopic features (Fig. 12.2)
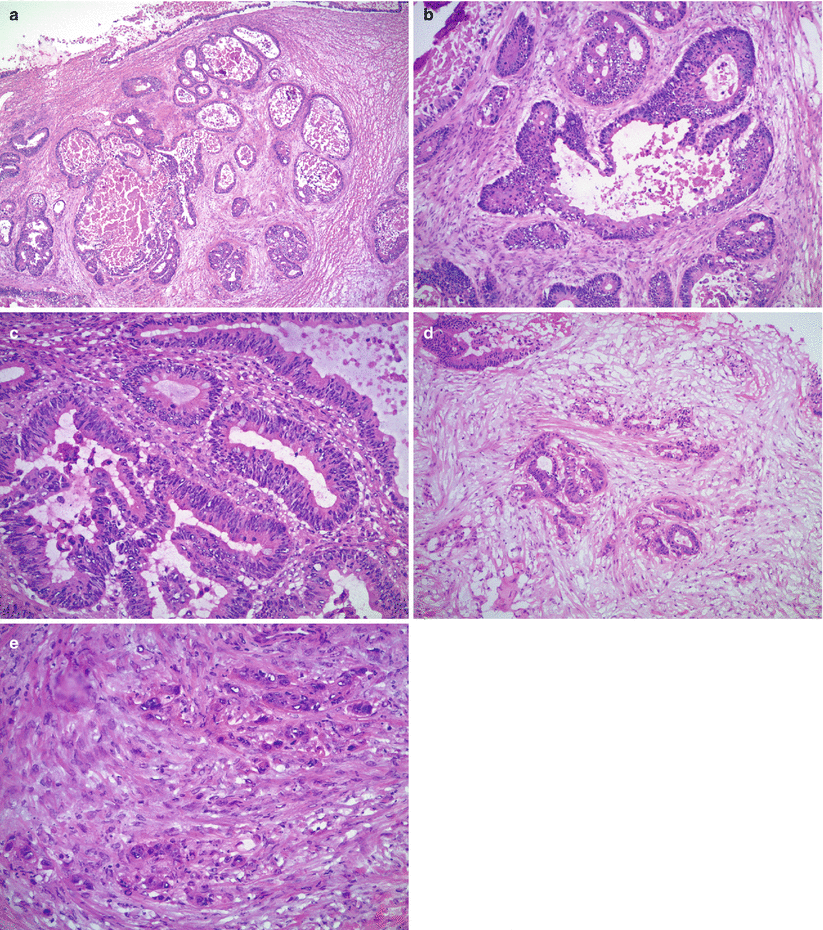
Fig. 12.2
Metastatic colorectal adenocarcinoma. Note the variably sized, irregular, and cystically dilated glands (a) with intraluminal necrotic debris (b) and moderate to severe nuclear atypia (c). Goblet cells are present but often are few in number (c). Stromal infiltration with desmoplastic reaction (d) and single cell infiltrative pattern (e) are helpful microscopic features
Variably sized, often cystic, irregular glands.
Cribriform architecture may be seen.
“Dirty necrosis”—eosinophilic necrotic debris with karyorrhexis—within the gland lumens or surrounded by glandular epithelium in a garland pattern.
Moderate to severe nuclear atypia.
Rare mucin-containing goblet cells.
Infiltrative growth at least focally with desmoplastic stromal reaction.
Differential diagnosis
Primary ovarian mucinous adenocarcinoma
Primary ovarian endometrioid adenocarcinoma
Sertoli-Leydig cell tumor with mucinous heterologous elements
Diagnostic pitfalls/key intraoperative consultation issues
The ovarian metastasis may precede the diagnosis of colonic primary.
Patients presenting with ovarian metastasis first tend to be younger compared with those who have a known colonic primary (mean age 48 versus 61 years, respectively) [10].
Bilaterality, size <10 cm, ovarian surface involvement, multinodular growth pattern, and infiltrative growth with desmoplastic stromal reaction favor metastasis over ovarian primary (see Chap. 8, Table 8.1).
Metastatic colorectal carcinomas may have a functioning ovarian stroma with androgen or estrogen production.
Distinction between primary ovarian mucinous adenocarcinoma and metastatic colorectal carcinoma may not be possible at the time of frozen section especially in the absence of a known primary.
It is important to correctly identify mucinous differentiation and raise the possibility of a metastasis, as it gives the opportunity for thorough intraoperative surgical evaluation of the abdominal cavity—including intestines—and possibly additional sampling.
Metastatic intestinal/colorectal carcinoma may also mimic primary endometrioid adenocarcinoma of the ovary.
Most helpful distinctive microscopic feature of endometrioid adenocarcinoma is squamous differentiation, which is absent in intestinal-type adenocarcinomas.
If only one ovary is sent for frozen section, the surgeon should be inquired about the appearance of the other ovary and the possibility of bilaterality.
Association with mature teratoma favors ovarian primary.
Metastatic Adenocarcinoma with Signet-Ring Cells/Krukenberg Tumor
Clinical and gross features
The primary tumor is located in the stomach (often pylorus) in approximately 70 % of cases, less commonly in the colorectum, appendix, breast, and pancreatobiliary system [6, 11].
Often presents in young patients, the average age is 45 years [11].
May have functioning ovarian stroma resulting in endocrine manifestations.
Other symptoms may include abdominal swelling or pain.
Ascites has been reported in over 40 % of cases [11].
At least 80 % of tumors are bilateral [6].
Average tumor size is 10.4 cm, with most tumors falling between 5 and 20 cm [11].
Irregular ovarian surface, often involved by tumor.
Cut surface is most often solid, tan-yellow or white, and multinodular; may also be firm or soft and gelatinous—depending on the mucin content and proportion of fibrous stroma (Fig. 12.3).
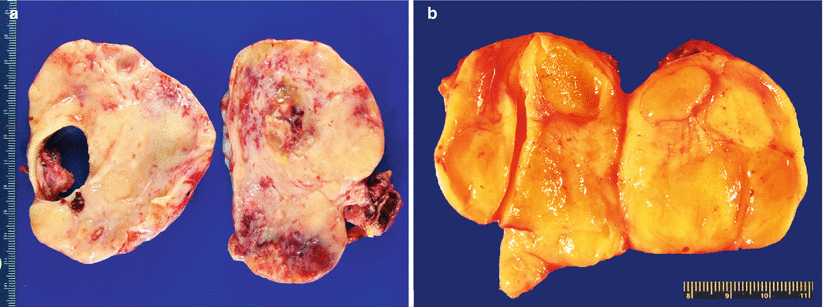
Fig. 12.3
Krukenberg tumor/metastatic signet-ring cell carcinoma (a, b). Note the characteristic solid, tan-yellow, multinodular, and often gelatinous (b) gross appearance
Hemorrhage may be seen; necrosis is not common.
Microscopic features (Fig. 12.4 stomach, Fig. 12.5 colon, Fig. 12.6 breast)
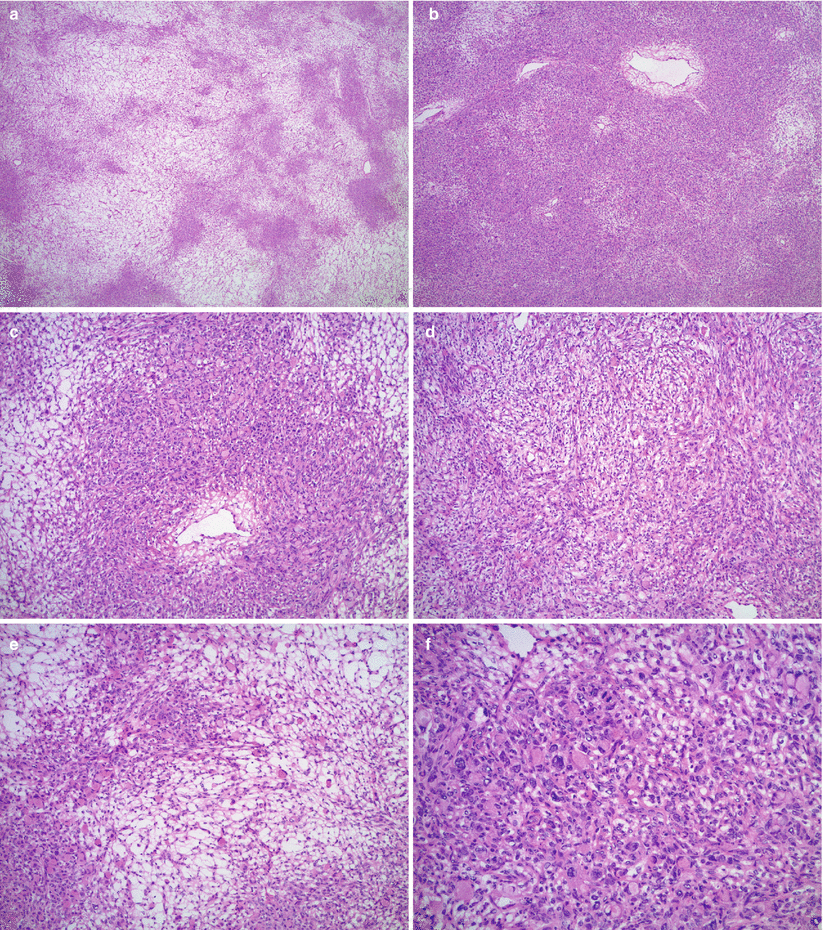
Fig. 12.4
Krukenberg tumor/metastatic signet-ring cell carcinoma from a gastric primary. Low magnification reveals a pseudo-lobular pattern with alternating hypo- and hypercellular areas (a). Densely cellular ovarian stroma may mimic an ovarian stromal tumor (b). Scattered individual tumor cells with relatively bland, small, eccentrically located nuclei and abundant pale basophilic or eosinophilic cytoplasm infiltrate the stroma (c–e). Less often marked nuclear atypia and brisk mitotic activity may be seen (f)
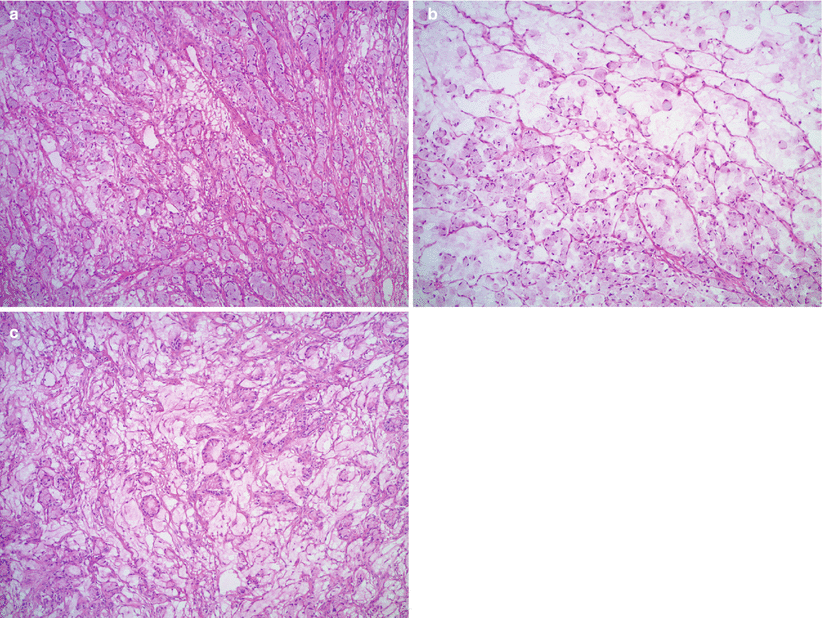
Fig. 12.5
Krukenberg tumor/metastatic signet-ring cell carcinoma from a colonic primary (a–c). Signet-ring cells are forming small clusters and tubules. Abundant extracellular mucin is also present
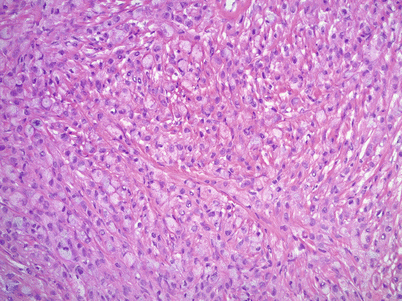
Fig. 12.6
Metastatic signet-ring cell carcinoma from a breast primary. Metastatic lobular carcinoma of the breast may have signet-ring cell morphology infiltrating the ovarian stroma in single cell files
Low magnification may show a pseudo-lobular pattern with alternating hyper- and hypocellular—edematous—areas or diffusely cellular dense stroma.
Signet-ring cells infiltrate individually, or form small clusters, diffuse sheets or small tubular structures.
Eccentric nuclei with abundant basophilic, eosinophilic, or clear cytoplasm
Variable nuclear pleomorphism and mitotic activity, but most often only mild atypia and relatively low mitotic rate
Extracellular mucin pools may be present.
Stroma may be prominent and may contain luteinized cells.
Differential diagnosis
Benign stromal tumors of ovary: fibroma, cellular fibroma, thecoma, sclerosing stromal tumor, and signet-ring stromal tumor
Stromal hyperplasia/hyperthecosis
Sertoli-Leydig cell tumor (versus tubular Krukenberg tumor)
Goblet cell (mucinous) carcinoid
Diagnostic pitfalls/key intraoperative consultation issues
Signet-ring cells may be missed on low magnification in the background of a densely cellular stroma.
High magnification assessment is recommended before the diagnosis of fibroma/fibrothecoma is made, to rule out metastatic signet-ring cell carcinoma.
Krukenberg tumors are very often bilateral (~80 % of cases), whereas bilaterality is rare among benign ovarian stromal and sex cord—stromal tumors; only approximately 8 % of ovarian fibromas are bilateral.
Pancreatobiliary Adenocarcinomas
Clinical and gross features
Relatively uncommon metastatic tumor in the ovary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


