Diabetes Mellitus
Diabetes mellitus (DM) is the term used to describe a group of metabolic disorders characterised by hyperglycaemia and due to:
- deficiency in pancreatic beta (β)-cell insulin production; and/or
- impaired insulin action (typically due to insulin resistance).
Diagnostic criteria
The World Health Organization (WHO) Expert Committee on the Diagnosis and Classification of Diabetes Mellitus recommends that DM should be diagnosed in the following circumstances:
- symptoms of diabetes and random venous plasma glucose ≥ 11.1 mmol/l;
- fasting venous plasma glucose (FPG) ≥ 7.0 mmol/l (confirmed on a second occasion in asymptomatic individuals).
The term impaired fasting glycaemia (IFG) has also been introduced for those with fasting venous plasma glucose levels in the range 6.1–6.9 mmol/l. The WHO recommends that patients in the latter category should proceed to an oral glucose tolerance test (OGTT):
- Venous plasma glucose ≥ 11.1 mmol/l (2 h after 75 g of oral anhydrous glucose) confirms a diagnosis of DM; those with 2-h venous plasma glucose levels in the range 7.8–11.0 mmol/l are deemed to have impaired glucose tolerance (IGT).
The American Diabetes Association (ADA) has recently accepted the addition of haemoglobin A1c (HbA1c) ≥ 6.5% as sufficient criteria for a diagnosis of DM (with HbA1c values in the range 5.7–6.4% denoting ‘prediabetes’); however, there is considerable on-going debate regarding this, and the WHO position is currently awaited.
Classification
Diabetes mellitus can be broadly classified into type 1 and type 2, although not all patients are easily assigned to one or other category. In addition, there are other specific types of diabetes (e.g. gestational DM and diabetes due to specific genetic defects), which are best considered independently (Table 17.1).
Table 17.1 Classification of Diabetes Mellitus
| Classification | Examples (list is not exhaustive) |
| Type 1 DM | |
| Type 2 DM | |
| Gestational DM | |
| Other specific types | |
| Monogenetic disorders | MODY |
| MELAS | |
| Lipodystrophy* | |
| Leprechaunism† | |
| Diseases of exocrine pancreas | Pancreatitis |
| Haemochromatosis | |
| Cystic fibrosis | |
| Neoplasia | |
| Trauma/surgery | |
| Endocrine disorders | Acromegaly |
| Cushing syndrome | |
| Phaeochromocytoma | |
| Glucagonoma | |
| Drug-induced | Corticosteroids Thiazide diuretics |
| Olanzapine | |
| Alpha interferon | |
| Conditions associated with DM | Down syndrome |
| Kilnefelter syndrome | |
| Turner syndrome | |
| Laurence–Moon–Biedl syndrome | |
| Prader–Willi syndrome | |
| DM, diabetes mellitus; MELAS, mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes; MODY, maturity-onset diabetes of the young. | |
| * Lipodystrophy may be caused by mutations in several different genes. | |
| † Leprechaunism is linked to defects in insulin receptor signalling. | |
Type 1 Diabetes Mellitus
This was previously referred to as insulin-dependent diabetes mellitus (IDDM).
- An autoimmune disorder in which the insulin-producing β-cells of the pancreas are destroyed; hence there is absolute insulin deficiency.
- Although type 1 DM develops mostly during childhood and adolescence, it may also present in later life.
- Patients typically experience an acute onset of the disease (weeks rather than months) and often give a history of significant weight loss. They are dependent upon insulin therapy and are prone to ketoacidosis (see below).
Type 2 Diabetes Mellitus
This was previously referred to as non-insulin-dependent diabetes mellitus (NIDDM).
- The predominant form worldwide, accounting for ~90% of patients with DM.
- Tissue insensitivity to insulin action (i.e. insulin resistance), and an inability of the pancreatic β-cells to compensate adequately for this, leads to overproduction of glucose by the liver and under utilisation by other tissues, with an inevitable rise in blood glucose levels – i.e. there is relative insulin deficiency.
- Traditionally this form of DM was considered predominantly a disease of obese, older adults, although now it is being increasingly diagnosed in adolescents and younger adults (and even in some children) due to the increasing prevalence of obesity in these age groups.
- Symptoms are often mild in the early stages, which can lead to a delay in the patient seeking medical attention; hence, diagnosis may occur later in the disease process, with some cases presenting with established complications (e.g. retinopathy, neuropathy or cardiovascular disorders).
- There may be a history of recurrent infections and injuries that are slow to heal.
- Ketosis is uncommon, except in situations of extreme stress, as patients usually have sufficient insulin to prevent lipolysis.
- Although initially controlled with diet and/or oral hypoglycaemic agents, many patients eventually need supplemental insulin.
- Type 2 DM is recognised as part of the so-called metabolic syndrome, where it is associated with central obesity, hypertension, dyslipidaemia (low HDL cholesterol and hypertriglyceridaemia – see later) and premature cardiovascular disease.
Gestational diabetes mellitus (GDM)
Most women who develop DM during pregnancy have normal glucose homeostasis during the first half of gestation, but develop a relative insulin deficiency during the second half, leading to hyperglycaemia. The guidelines published in March 2010 by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommends that all women without a prior diagnosis of DM be considered for a 75 g anhydrous glucose OGTT at 24–28 weeks’ gestation. If ≥ two of the following criteria are met, then the woman is deemed to have a diagnosis of GDM:
- fasting venous plasma glucose ≥ 5.3 mmol/l
- 1 h venous plasma glucose level ≥ 10.0 mmol/l
- 2 h venous plasma glucose level ≥ 8.6 mmol/l.
The UK National Institute for Health and Clinical Excellence (NICE) guidance (2008), however, only advises screening the following ‘at risk’ groups:
- BMI ≥ 30 kg/m2
- previous macrosomic baby (weighing ≥ 4.5 kg)
- previous GDM
- first-degree relative with DM
- ethnic origin with a high prevalence of DM (South Asian, black Caribbean and Middle Eastern).
If screen positive (i.e. one positive risk factor), offer 75 g OGTT at 28 weeks. If previous GDM, offer early self-monitoring of blood glucose or early OGTT at 16 weeks, repeating at 24 weeks if initially negative.
Currently, NICE recommends GDM be diagnosed if fasting venous plasma glucose ≥ 7 mmol/l or if 2 h venous plasma glucose in an OGTT is ≥ 7.8 mmol/l.
Other Specific Types of Diabetes Mellitus
This category includes a diverse spectrum of conditions, ranging from rarer genetic disorders to primary pancreatic pathology, endocrine diseases and drug-induced DM (Table 17.1).
Epidemiology
According to the WHO, the number of adults with DM worldwide was estimated to be 171 million in 2000 and is expected to rise to 366 million by 2030. The prevalence of DM in the UK is approximately 4% and has more than doubled since 1991.
Aetiology
Genetics
Type 1 DM
The identical twin of a person with type 1 DM has a 30–50% chance of developing the disease, implicating both genetic and environmental factors in its aetiology. A number of genetic susceptibility loci have been identified and include:
- IDDM1 in the major histocompatibility complex (MHC)/human leucocyte antigen (HLA) region on chromosome 6p21.3.
- IDDM2 near the insulin gene locus on chromosome 11p15.
Overall, the risk of a sibling or offspring of an individual with type 1 DM developing the same condition is relatively low but increased compared to the background population. The risk is higher if the father has the condition (6–8% versus 2–4% if the mother is affected).
Type 2 DM
Typically, there is a positive family history. In most affected individuals the inherited component is likely to be polygenic, involving interaction between multiple genes involved in both insulin secretion and insulin action. Overall, the risk of a sibling or offspring of a person with type 2 DM developing the condition is high (as much as 33%; identical twins are affected in 60–100% of cases). Lifestyle changes can modify this risk (see below).
MODY
MODY is an autosomal dominant condition. Individual subtypes are associated with mutations in a variety of different genes that encode factors involved in insulin production/release by pancreatic β-cells. For example, MODY type 2 is caused by mutations in the gene coding for glucokinase, a rate-limiting enzyme in the glycolytic pathway, which acts as a pancreatic ‘glucose sensor’, regulating insulin release in response to a rise in blood glucose levels. Mutations in glucokinase are associated with an altered ‘set-point’ for glucose sensing, i.e. insulin release is triggered at higher ambient blood glucose levels compared with the general population. Accordingly, hyperglycaemia tends to be mild and relatively stable over time, and is often detected incidentally or as part of family screening; severe hyperglycaemia and complications are rare. In contrast, in MODY type 3, mutations in a transcription factor, hepatocyte nuclear factor 1α (HNF1α), which regulates β-cell mass/insulin production, result in DM that typically presents in adolescence or young adulthood, with progressive hyperglycaemia over time such that treatment with oral hypoglycaemic agents and/or insulin is usually required. Patients with MODY 3 are particularly sensitive to the blood glucose lowering effects of sulphonylureas (see below), and patients started on insulin therapy prior to the diagnosis being made may be able to transition back to a sulphonylurea with maintenance of excellent glycaemic control; however, many patients ultimately require insulin therapy. Currently, HNF1α mutations account for  70% of all MODY cases.
70% of all MODY cases.
MELAS
MELAS is a rare disorder characterised by mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes. It is due to mutations in genes encoded by mitochondrial DNA (e.g. MT-TL1 and MT-ND1) and maternally inherited.
Environmental/Other Factors
Type 1 DM
In genetically susceptible individuals, one or more environmental factors may ‘trigger’ immune-mediated destruction of islet β-cells (‘insulinitis’). A variety of agents have been implicated including viruses (e.g. coxsackie, rubella, mumps, cytomegalovirus), dietary constituents (e.g. bovine serum albumin in cow’s milk, especially if fed to infants) and stress.
Type 2 DM
This is strongly linked to obesity, which predisposes to insulin resistance. Fetal malnutrition in utero may also be associated with an increased risk (so-called fetal programming).
Clinical Presentation
The classic triad of diabetic symptoms include:
- polyuria
- increased thirst (polydipsia)
- weight loss.
These features typically manifest in an acute or subacute fashion in those with type 1 DM, but may be of gradual onset in the setting of type 2 DM. Other presenting features include:
- tiredness/fatigue
- blurred vision
- opportunistic infections (e.g. balanitis, thrush, pruritus vulvae).
Individuals with type 1 DM are traditionally thought of as being ‘lean’ as opposed to their type 2 DM counterparts, who are often overweight/obese. However, with the rising ‘obesity epidemic’ the margins have begun to ‘blur’ as more obese type 1 cases are identified. Similarly, not all patients with type 2 DM are necessarily obese.
Occasionally, diabetes may present as part of another disorder (e.g. Cushing syndrome, acromegaly, haemochromatosis) where features of the primary condition dominate the clinical picture, or may be detected incidentally or during screening (e.g. on urinalysis or a fasting/random blood sample).
Once the clinical diagnosis of DM is suspected, it is usually relatively easily confirmed (see diagnostic criteria above). In cases where there is difficulty in distinguishing between type 1 and type 2 DM, measurement of plasma C-peptide (low/absent in type 1 DM; present/elevated in type 2 DM) and screening for islet cell and anti-GAD (glutamic acid decarboxylase) antibodies may be of help.
Management
General Principles
The major aim of management is to achieve near normal glucose homeostasis (for which patient education is a key component), initially to provide symptom relief and in the longer term to prevent/minimise complications – surveillance for, and treatment of, the latter is also a priority. Management should be coordinated by a specialist multidisciplinary diabetes team, based in either primary or secondary care, and individuals with DM should be reviewed at least once, and ideally twice, a year by this team. Table 17.2 shows aspects of the patient’s care that should be assessed during each ‘annual review’ process.
Table 17.2 Key Elements of the Diabetic ‘Annual Review’ Process
| Element | Comment(s) |
| Diet and eating habits | Promote healthy diet/eating habits with specific advice pertaining to DM |
| Weight | Encourage maintenance of optimal weight |
| Physical activity | Explain that exercise improves glycaemic control, blood pressure and lipid profile |
| Glycaemic control | Assess by reviewing home blood glucose diary/meter in conjunction with HbA1c |
| Hypoglycaemia | Check: frequency; awareness; ability to recognise and treat appropriately |
| Complications: | |
| retinopathy | Confirm enrolment into retinal photography screening programme; ask about visual change/symptoms |
| nephropathy | Check serum creatinine and urine ACR |
| neuropathy | Enquire about symptoms and examine for evidence of impaired sensation |
| foot problems | As for neuropathy, but also check: peripheral pulses; for callus, ulceration and Charcot arthropathy |
| cardiovascular disease | Ask about/examine for features of IHD; stroke/TIA; peripheral vascular disease |
| Associated risk factors | Advise avoidance of/cessation from smoking; ensure within targets for blood pressure and lipids (see text) |
| Medications | Check: DM treatment regimen, antihypertensive/renal protection therapy, lipid-lowering agents and, where appropriate, antiplatelet therapy |
| Injection sites | Look for evidence of lipohypertrophy (or rarely lipoatrophy) and advise rotation of injection sites |
| Driving | Ensure awareness of DVLA regulations/advice (e.g. test blood glucose before driving – should be > 5 mmol/l) |
| Alcohol | Advise variable impact on blood glucose – mainly risk of late hypoglycaemia and possible requirement for adjustment to insulin regimen |
| Sexual function | Ask about erectile dysfunction in men; advise on contraception and preconception glycaemic control in women |
| Psychological factors | Check for concerns, e.g. regarding coping with a chronic illness |
| Blood tests | Electrolytes, renal function, liver function, glucose, HbA1c, fasting lipid profile, full blood count |
| ACR, albumin : creatinine ratio; DM, diabetes mellitus; DVLA, Driver Vehicle and Licensing Authority; HbA1c, haemoglobin A1c; IHD, ischaemic heart disease; TIA, transient ischaemic attack. | |
In addition to the diabetologist/GP with a specialist interest, diabetes specialist nurse/practice nurse and dietician, patients with diabetes may also require input from the following disciplines: chiropody, psychology, orthopaedics, vascular surgery, ophthalmology and urology.
A plethora of clinical trials have confirmed the benefits of achieving/maintaining good glycaemic control in DM, but two landmark studies merit particular mention: the Diabetes Control and Complications Trial (DCCT – type 1 DM) and the United Kingdom Prospective Diabetes Study (UKPDS – type 2 DM) – see Box 17.1.
 Box 17.1 Glycaemic control in diabetes – landmark trials
Box 17.1 Glycaemic control in diabetes – landmark trials- Intensive treatment reduced the risk of developing retinopathy by 76% in the primary prevention group, and of worsening retinopathy by 54% (and of developing proliferative or severe non-proliferative retinopathy by 47%) in the secondary prevention group.
- In the two groups combined, intensive therapy reduced the risk of developing microalbuminuria by 39%, albuminuria by 54% and neuropathy by 60%.
- The major adverse event in the intensively treated group was a two- to threefold increase in severe hypoglycaemic episodes.
- Retinopathy, nephropathy and possibly neuropathy are benefited by lowering blood glucose levels in type 2 DM with intensive therapy, which achieved a median HbA1c of 7.0% compared with conventional therapy with a median HbA1c of 7.9%.
- The overall microvascular complication rate was decreased by 25%, and for every percentage point decrease in HbA1c there was a 35% reduction in the risk of complications; there was no evidence of any glycaemic threshold for any of the microvascular complications above normal glucose levels.
- A 16% reduction (which was not statistically significant, p = 0.052) in the risk of combined fatal or non-fatal myocardial infarction and sudden death was observed.
- For every percentage point decrease in HbA1c, there was a 25% reduction in diabetes-related deaths, a 7% reduction in all-cause mortality, and an 18% reduction in combined fatal and non-fatal myocardial infarction. Again no glycaemic threshold for these macrovascular complications was observed.
- Lowering blood pressure to a mean of 144/82 mmHg significantly reduced strokes, diabetes-related deaths, heart failure, microvascular complications and visual loss.
- A log-linear relationship between the incidence of complications and increasing HbA1c or systolic blood pressure indicated that any improvement in glycaemic or blood pressure control would be advantageous.
- Metformin appeared to confer particular benefits in obese subjects with T2DM, and was associated with less weight gain and fewer hypoglycaemic episodes.
DM, diabetes mellitus; FPG, fasting plasma glucose; HbA1c, haemoglobin A1c.
Glycaemic Control
Monitoring
This is typically assessed using a combination of the patient’s home blood glucose monitoring (HBGM) records and glycosylated haemoglobin A1c (HbA1c) measurement. The latter provides an estimate of overall glycaemic control during the past 6–8 weeks, and can be assayed either using a near-patient testing kit or in the clinical biochemistry laboratory. Due to potentially significant variations in analytical methods and quality control, all assays have traditionally been aligned to that used in the DCCT and expressed as a percentage. However, a new standard has recently been introduced by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), which expresses HbA1c values in mmol/mol of unglycosylated haemoglobin. Table 17.3 shows DCCT and IFCC equivalents.
Table 17.3 Diabetes Control and Complications Trial (DCCT) and International Federation of Clinical Chemistry (IFCC) equivalent HbA1c values
| DCCT-aligned HbA1c (%) | IFCC-standardised HbA1c (mmol/mol) |
| 4.0 | 20 |
| 5.0 | 31 |
| 6.0 | 42 |
| 6.5 | 48 |
| 7.0 | 53 |
| 7.5 | 59 |
| 8.0 | 64 |
| 8.5 | 69 |
| 9.0 | 75 |
| 9.5 | 80 |
| 10.0 | 86 |
The frequency/necessity of HBGM continues to be widely debated. At a minimum, testing should be undertaken during periods of change or intercurrent illness, and in all patients on insulin therapy, with recommendations determined on an individual patient basis. Continuous glucose monitoring systems (CGMS) are available, but are expensive and generally reserved for ‘difficult cases’ and/or highly engaged individuals.
Similarly, ‘ideal’ blood glucose targets are often debated and ‘one size does not fit all’; in general, HBGM levels of 4–8 mmol/l are likely to correlate with a satisfactory HbA1c (< 7.0%; 53 mmol/mol). However, individualised targets should be agreed between the patient and his/her clinician, bearing in mind the large volume of trial data showing that good glycaemic control reduces the risk of microvascular and macrovascular complications, and that in some settings (e.g. pregnancy – see below) tight glycaemic control is particularly important (to reduce the risk of fetal malformations and macrosomia), but that for others (e.g. the frail/elderly living alone) the need to avoid hypoglycaemia may necessitate running the HbA1c at less than optimal levels.
Treatment of Hyperglycaemia
Type 1 DM
Patients with type 1 DM require insulin to control their blood glucose levels. Education about the effects of diet, physical activity and illness upon glycaemic control, and hence insulin requirements, is critical.
Insulin Preparations
Based on source, insulins are classified as animal (porcine, bovine), human or analogue. The use of animal insulins has fallen in recent years, but there are still a small number of patients who derive particular benefits from these preparations. Based on the duration of action, insulin can be classified as rapid-, short-, intermediate- or long-acting. In addition, various mixed preparations exist, containing different proportions of faster- and slower-acting insulins; for example, Novomix-30 is a pre-mixed insulin analogue which contains 30% soluble insulin aspart (rapid-acting form) and 70% insulin aspart protamine (intermediate-acting form) (Tables 17.4 and 17.5).
Table 17.4 Classification of Insulin Preparations
| Type of insulin (with examples) | Source | Characteristics |
| Rapid-acting | ||
| Insulin aspart, insulin glulisine, insulin lispro | Analogue | Onset of action: within 15 min |
| Peak action: 1 h | ||
| Duration of action: 4 h | ||
| Short-acting/neutral | ||
| Actrapid®, Humulin S®, Insuman® rapid | Human | Onset of action: 30 min |
| Peak action: 1–2 h | ||
| Duration of action: 5–6 h | ||
| Hypurin® bovine neutral, Hypurin® porcine neutral | Animal | Onset of action: 30 min |
| Peak action: 2–4 h | ||
| Duration of action: 5–6 h | ||
| Intermediate/long-acting | ||
| Humulin I®, Insulatard®, Insuman® basal | Human | Onset of action: 30–60 min |
| Peak action: 4–8 h | ||
| Duration of action: 16– 20 h | ||
| Hypurin® bovine isophane, Hypurin® bovine lente, Hypurin® porcine isophane | Animal | Onset of action: 60–120 min |
| Peak action: 6–12 h | ||
| Duration of action: 16–20 h | ||
| Long-acting | ||
| Insulin detemir, insulin glargine | Analogue | Onset of action: 60–70 min |
| Peak action: no peak, flat profile | ||
| Duration of action: 20–24 h | ||
| Hypurin bovine protamine zinc insulin (PZI) | Animal | Onset of action: 240–600 min |
| Peak action: 10–20 h | ||
| Duration of action: 20–30 h | ||
Table 17.5 Types of Mixed/Biphasic Insulins
| Examples | Source | Characteristics |
| Humulin M3®, Insuman® Comb 15, Insuman® Comb 25, Insuman® Comb 50 | Human | Onset of action: 30 min |
| Peak action: 1–8 h | ||
| Duration of action: up to 20 h | ||
| Hypurin porcine 30/70 mix | Animal | Onset of action: 30 min |
| Peak action: 4–12 h | ||
| Duration of action: up to 22 h | ||
| Humalog® Mix25, Humalog® Mix50, NovoMix® 30 | Human | Onset of action: within 15 min |
| Peak action: 30–240 min | ||
| Duration of action: up to 20 h |
Insulin administration
Options include:
- syringe and needle
- ‘pen devices’
- insulin pump/continuous subcutaneous insulin infusion (CSII) therapy.
Insulin Regimens
A variety of different insulin regimens exist (Table 17.6).
Table 17.6 Insulin Regimens
| Regimen | Example | Indication(s) |
| Once-a-day | Insulin detemir, insulin glargine | Type 2 DM together with oral hypoglycaemic agents |
| Twice-a-day | Mixed/biphasic insulins | Type 2 (or type 1) DM |
| Thrice-a-day | Mixed insulin (morning), rapid acting (evening), long acting (night) or mixed biphasic insulin thrice daily | Used mostly in children to avoid the need to inject while at school; rarely used in adults (e.g. if high total daily insulin requirement) |
| Four times a day (‘basal-bolus’) | Long-acting insulin once (or occasionally twice) daily and rapid-acting insulin with each meal | Preferred regimen in type 1 DM; also used for young type 2 DM failing oral hypoglycaemic agents and once daily insulin |
Patient Education
Accredited evidenced-based programmes have now been developed, and the most widely recognised is ‘DAFNE’ (Dose Adjustment For Normal Eating). DAFNE offers a comprehensive programme designed to deliver the specific knowledge required by patients with type 1 DM to manage their daily insulin requirements based on diet, activity, illness, etc.
Other Therapeutic Options
A small number of patients may ultimately be considered for whole organ (pancreas – often combined with kidney) or islet cell transplantation, but currently these options are limited and only available in a small number of centres.
Type 2 DM
Patient Education
Type 2 DM may be prevented, or at least its onset delayed, by making significant lifestyle changes in those at high risk of the condition. Dietary modification and physical activity are critically important for achieving good glycaemic control. A comparable programme to DAFNE for type 2 DM – DESMOND (Diabetes Education and Self-Management for ONgoing and Diagnosed) is recommended by NICE.
If these measures alone fail to control blood glucose levels, then oral hypoglycaemic agents are typically commenced. Ultimately, many patients require combination therapy with oral hypoglycaemic agents and/or injectable therapies (e.g. incretin mimetics or insulin – see below).
Table 17.7 shows the oral hypoglycaemic agents currently available in the UK.
Biguanides
Metformin, the only biguanide licensed in the UK, reduces hepatic glucose production, mainly by inhibiting gluconeogenesis. It also increases peripheral insulin sensitivity, albeit through a poorly understood mechanism. Beneficial effects include promotion of weight loss, reductions in total and low-density lipoprotein (LDL) cholesterol and triglycerides.
Gastrointestinal side effects are common, especially during the early stages of treatment, but usually subside (hence the mantra ‘low (dosage) and slow (dosage increments)’). It should be avoided in patients with renal impairment (serum creatinine > 150 mmol/l or GFR < 30 ml/min) to reduce the risk of lactic acidosis. In addition, its use should be suspended in those undergoing radiological contrast studies or general anaesthesia and not restarted until renal function has returned to baseline.
Vitamin B12 deficiency may develop as a result of decreased absorption – annual full blood count is therefore recommended.
Sulphonylureas (Sulfonylureas)
Sulphonylureas augment residual pancreatic β-cell function, hence the term ‘insulin secretagogues’ (Table 17.7). They are licensed for monotherapy (mainly used in patients who are intolerant of metformin or in whom there is a specific indication for sulphonylurea therapy, e.g. MODY 3 (see earlier)), or in combination with other oral hypoglycaemic agents or insulin. Sulphonylureas are generally not recommended for use during pregnancy, although some clinicians believe that glibenclamide (US approved name glyburide) can be used safely after the first trimester.
Their major side effects are hypoglycaemia (especially in the elderly) and weight gain.
Prandial Glucose Regulators (‘Meglitinides’)
Repaglinide and Nateglinide are rapid-acting insulin secretagogues with a fast onset and short duration of action. They should be taken 15–30 min before each main meal. However, they are relatively expensive and although there are theoretical advantages to their use, in practice they offer few benefits over sulphonylureas.
Thiazolidinediones (TZDs; ‘Glitazones’)
The TZDs are high affinity ligands for the nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ), which is expressed ubiquitously, but at particularly high levels in adipose tissue.
Both pioglitazone and rosiglitazone are known to cause fluid retention and are contraindicated in those with/at risk of heart failure – this tendency is exacerbated when used in conjuction with insulin therapy. In addition, significant concerns have been raised regarding a possible increased risk of adverse ischaemic coronary events/outcomes in patients taking rosiglitazone and, after a series of reviews/updates to the product licence, eventually the marketing authorisation for rosiglitazone was suspended by the European Medicines Agency in September 2010. At the same time, the US Food and Drug Administration (FDA) placed significant restrictions on the use of this drug. Currently therefore, pioglitazone is the only TZD available for use in the UK.
Other adverse effects of TZD therapy include weight gain, increased fracture rate at peripheral sites in women and, rarely, hepatic dysfunction; in addition, specific concerns have been raised regarding an increased risk of bladder cancer in those taking pioglitazone.
Alpha-Glucosidase Inhibitors
These agents inhibit the function of intestinal alpha-glucosidases, thereby delaying the digestion and absorption of complex carbohydrates (e.g. starch, sucrose). Currently, only one agent, acarbose, is licensed for use in the UK. It has a relatively small effect on lowering blood glucose levels and is associated with an unwelcome side effect – flatulence!
Dipeptidylpeptidase-4 Inhibitors (DPP-4 Inhibitors; ‘Gliptins’)
Glucagon-like peptide 1 (GLP-1), an incretin that is secreted from the intestinal L-cells in response to nutrients, promotes glucose-dependent insulin secretion from pancreatic β-cells, while simultaneously suppressing glucagon release. In addition, GLP-1 slows gastric emptying and may confer additional ‘protective effects’ on the β-cells, through as yet unclear mechansims. Endogenous GLP-1 has a very short half-life in the circulation and is rapidly degraded by the enzyme dipeptidylpeptidase-4 (DPP-4). Accordingly, inhibitors of DPP-4 (e.g. saxagliptin, sitagliptin and vildagliptin) enhance endogenous GLP-1 signalling and are of potential benefit in the treatment of type 2 DM. DPP-4 inhibitors are generally weight neutral. Hypoglycaemia is relatively uncommon.
Glucagon-Like Peptide 1 (GLP-1) Agonists/Analogues (‘Incretin Mimetics’)
Exenatide and liraglutide are the first agents to become available in this class, and mimic the effects of endogenous GLP-1. Both are given by subcutaneous injection. Unlike the DPP-4 inhibitors, they promote weight loss, which has been postulated to occur via a variety of mechanisms, including delayed gastric emptying, which results in early satiety, and central appetite suppressant effects. Weight loss may be profound with a resultant reduction in insulin resistance to such an extent that other oral hypoglycaemics can be reduced/withdrawn. The risk of hypoglycaemia is generally low, which is advantageous for those occupations where insulin use is prohibited. However, when used in combination with sulphonylureas (see below), there is a significant increase in the risk of hypoglycaemia, and patients should be advised of this.
Gastrointestinal side effects, particularly nausea, are common; severe (rarely fatal) pancreatitis has also been reported and patients should be carefully counselled and advised of symptoms to report.
Insulin
The natural history of type 2 DM with insulin resistance is progressive pancreatic β-cell failure; so while good glycaemic control may initially be achievable through lifestyle measures alone, eventually most patients progress to require oral hypoglycaemic agents followed by incretin mimetics or insulin. Unfortunately, the use of insulin in type 2 DM is often complicated by weight gain. The different types of insulin regimen used in type 2 DM are shown in Table 17.6.
Diabetic Emergencies
There are two common types of diabetic coma:
- hypoglycaemic (the most common)
- hyperglycaemic (diabetic ketoacidosis (DKA) or hyperosmolar non-ketosis (HONK)).
Hypoglycaemia (‘Hypo’)
Hypoglycaemia in the context of DM occurs in patients who are treated with insulin and, less commonly, in those receiving certain oral hypoglycaemic agents (Table 17.7). It reflects an imbalance between insulin and carbohydrate, the most frequent precipitants being inappropriate medication use, excess physical activity, decreased food intake, excess alcohol ingestion or a combination of factors.
Table 17.7 Oral Hypoglycaemic Agents and Non-Insulin-Based Injection Therapy for Type 2 Diabetes Mellitus
| Class | Comment(s) |
| Biguanides | Suppress basal hepatic gluconeogenesis (major contributor to fasting hyperglycaemia in type 2 DM); also reduce peripheral insulin resistance, thereby increasing glucose utilisation. |
| e.g. metformin | – preferred first-line agent in type 2 DM, especially in overweight/obese subjects |
| – does not cause hypoglycaemia. | |
| Sulphonylureas | Insulin secretagogues, which act on ATP-sensitive potassium channels in pancreatic β-cells to promote insulin secretion. |
| 1stgeneratione.g. chlorpropamide, tolbutamide | – rarely used due to less favourable side effect profile, e.g. long duration of action of chlorpropamide, which predisposes to hypoglycaemia |
| 2nd and 3rdgeneration | – more potent, but generally better tolerated than first generation agents |
| e.g. gliclazide, glimepiride, glipizide | – predispose to hypoglycaemia, especially in the elderly and those with renal/hepatic impairment. |
| Meglitinides | Insulin secretagogues, which bind to ATP-sensitive potassium channels on pancreatic β-cells in a similar manner to sulphonylureas, but at a discrete binding site. |
| e.g. nateglinide, repaglinide | – rapid onset and very short duration of action; controls prandial hyperglycaemia |
| – reduced risk of postabsorptive hypoglycaemia. | |
| Thiazolidinediones (TZDs) | Selective agonists of the nuclear receptor PPARγ, which improve peripheral insulin sensitivity and promote more favourable adipose tissue distribution/function, but at a cost of overall weight gain. |
| e.g. pioglitazone | – hypoglycaemia is relatively uncommon with monotherapy, but risk may be increased by concomitant use of certain other oral hypoglycaemic agents (especially sulphonylureas) or insulin. |
| Alpha-glucosidase inhibitors | Impair the enzymatic degradation of complex carbohydrates (e.g. starch and sucrose) in the small intestine, thereby delaying their digestion and absorption. |
| e.g. acarbose | – modest effect on lowering blood glucose |
| – not associated with hypoglycaemia when used alone. | |
| DPP-4 inhibitors | Inhibit the function of DPP-4, thus impairing the degradation of the endogenous incretin GLP-1, which leads to an increase in GLP-1 levels, thereby promoting insulin release and suppressing glucagon secretion. |
| e.g. saxagliptin, sitagliptin, vildagliptin | – hypoglycaemia is relatively uncommon. |
| GLP-1 agonists/analogues | Incretin mimetics, which mimic endogenous GLP-1 action, thereby increasing insulin secretion, suppressing glucagon secretion and slowing gastric emptying. |
| e.g. exenatide, liraglutide | – hypoglycaemia is a recognised albeit uncommon side effect. |
| ATP, adenosine triphosphate; DM, diabetes mellitus; DPP-4, dipeptidylpeptidase-4; GLP-1, glucagon-like peptide 1; PPARγ, peroxisome proliferator-activated receptor γ. | |
Hypoglycaemia is usually associated with the development of so-called warning symptoms – hypoglycaemic awareness involves:
- ‘Autonomic’ symptoms – a physiological response that is associated with the release of counter-regulatory hormones (adrenaline (epinephrine) and noradrenaline (norepinephrine)). Clinical signs include tremor, sweating, anxiety, palpitations and shivering.
- ‘Neuroglycopenic’ symptoms – manifest if hypoglycaemia is prolonged and more profound; as glucose is the only fuel source readily utilised by the brain, cognitive function is affected with symptoms of tiredness, dizziness, drowsiness, difficulty in speaking, inability to concentrate, confusion and aggression; as a general rule, neuroglycopenic symptoms develop when blood glucose is < 2.6 mmol/l; if left untreated coma and death may ensue.
Although the blood glucose level at which people begin to experience hypoglycaemic symptoms varies, the consensus for defining hypoglycaemia in the context of DM is a blood glucose value of < 4 mmol/l. ‘Four is the Floor’ has proved to be a successful awareness campaign.
Management
The Joint British Diabetes Societies Inpatient Care Group (JBDS IP Group) has published recommendations for the treatment of hypoglycaemia, with the aim of standardising treatment within the National Health Service (NHS):
- If the patient is conscious, advise 15–20 g of rapidly acting carbohydrate (e.g. 50 ml Lucozade™, 3 dextrose tablets) to correct the hypoglycaemia, always followed with longer acting/complex carbohydrate to maintain glucose in the normal range (this may be the next meal if imminent; if not, offer toast or a sandwich).
- If the patient is semiconscious, then apply commercially available glucose gel to the buccal mucosa; this may allow sufficient improvement in conscious level to continue treatment as per a conscious individual.
- If the patient is unconscious, administer 50 ml of 20% dextrose intravenously (50% dextrose may cause significant phlebitis) via a large vein, followed by a generous flush; if intravenous access is not available, then 1 mg of glucagon can be given intramuscularly while awaiting further medical help.
Ascertaining the cause of recurrent hypoglycaemia is often neglected by the individual, or clinician. It should be sought and remedial action taken. Sometimes early morning headaches or a restless night may be the only indication of nocturnal hypoglycaemia.
NB Recurrent and severe hypoglycaemia may cause a downregulation of the autonomic reponse to hypoglycaemia, resulting in reduced warning, so-called hypoglycaemic (‘hypo’) unawareness. Development of the latter has significant social/lifestyle implications, particularly with respect to driving.
Hyperglycaemia
Diabetic Ketoacidosis (DKA)
DKA most commonly occurs in the context of type 1 DM, but is also occasionally seen in type 2 DM under conditions of extreme stress. Glucose cannot be utilised as an energy substrate in the absence of insulin, and the body ‘perceives’ itself to be lacking in glucose, hence hepatic gluconeogenesis and glycogenolysis are enhanced, exacerbating the hyperglycaemia. An alternative energy substrate is sought, and lipolysis occurs with the mobilisation and increased production of fatty acids and amino acids; ketones are produced as a toxic metabolite of this process within the liver, resulting in the development of metabolic acidosis.
DKA is defined as a triad of:
- hyperglycaemia (plasma glucose > 11 mmol/l)
- acidosis (venous pH < 7.3)
- ketosis (either ketonaemia (> 3 mmol/l) and/or ketonuria (> 2+)).
The incidence, using all three criteria, is estimated at 4–8 episodes per 1000 diabetic individuals. It is a potentially life-threatening catabolic state, but fortunately mortality rates are now low in the developed world (< 1%). Life-threatening complications include cerebral oedema (remains the commonest cause of mortality in the young), hypokalaemia and the development of adult respiratory distress syndrome.
The two major precipitating factors for DKA are inadequate insulin therapy, either deliberate or accidental, and intercurrent infection. All patients with type 1 DM should be educated regarding the nature, cause and prevention of DKA, often referred to as the ‘sick day rules’.
Symptoms of DKA include thirst, dry mouth, polyuria, nausea, vomiting, weakness, myalgia, headache and abdominal pain. Drowsiness may progress to confusion and coma. It is rare for DKA to develop without a prodromal phase, and symptoms of hyperglycaemia have often been present for at least 24 h, but missed or ignored. Clinical signs include dehydration, tachycardia, hypotension, hyperventilation (Kussmaul breathing) and the characteristic smell of ketones. Hypovolaemic shock can ensue in more severe cases.
Management
The primary aim of treatment is to suppress ketogenesis, rather than normalise hyperglycaemia. The JBDS IP Group has released guidance on the management of DKA (March 2010). As with hypoglycaemia, the aims are to standardise care within the NHS, while at the same time incorporating recent advances in technology and recognising the changing presentation of DKA.
The major technological advance relates to near patient (i.e. bedside) routine testing of blood ketones (3-betahydroxybutyrate), and using the fall in ketonaemia as a guide to the response to treatment.
Recognition that ‘euglycaemic acidosis’ is now a more common presentation (with improved education, individuals often manage to partially treat their acidosis, often leading to lower blood glucose levels at presentation) is also emphasised in the guidance.
The management of DKA revolves around:
- fluid replacement
- insulin replacement
- metabolic treatment targets
- additional measures.
Fluid Replacement
It is universally acknowledged that the most important therapeutic intervention in DKA is the immediate administration of fluids. Crystalloid is the fluid of choice (0.9% sodium chloride) even in the hypotensive individual. Fluids are required to:
- Restore circulatory volume: the required rate of fluid infusion will vary depending on the age of the patient (children and young adults appear to be at increased risk of cerebral oedema from overexuberant fluid resuscitation, while otherwise previously fit adults generally tolerate rapid initial fluid replacement, e.g. first 1 l of 0.9% sodium chloride over 1 h, with the second and third litres over 2 h each, increasing to 4-hourly bags for the fourth and fifth litres, and then 6 hourly, with reassessment of cardiovascular status on a regular basis). Care must also be exercised in the elderly, during pregnancy and in patients with pre-existing renal or cardiac failure or other serious comorbidities.
- Enable clearance of ketones: with lower glucose levels at presentation, administration of intravenous insulin can lead to hypoglycaemia well before ketogenesis is fully suppressed; as it is imperative to continue the insulin infusion until the latter has been achieved, the administration of 10% glucose, often concurrently with 0.9% sodium chloride, is often required (most units would start intravenous glucose when blood glucose levels fall below 14 mmol/l).
- Correct electrolyte imbalance: potassium is the predominant intracellular cation and is actively transported into cells through a glucose/insulin-dependent channel; at presentation hyperkalaemia is common in DKA, as potassium cannot enter the cells in the absence of insulin; however, total body potassium is low and following the administration of intravenous insulin serum potassium levels plummet and regular monitoring is therefore mandatory.
NB Hypo- and hyperkalaemia are important causes of mortality in DKA.
The JBDS IP Group also provides simple guidance for the addition of potassium to intravenous fluids during the first 24 h of treatment:
- serum potassium > 5.5 mmol/l – none
- serum potassium 3.5–5.5 mmol/l – add 40 mmol/l
- serum potassium < 3.5 mmol/l – senior review as additional potassium is required and the patient may require transfer to a higher care setting to allow this to be given safely.
NB Caution must be exercised in patients with suspected renal failure, especially in those with poor/no urine output despite initial fluid resuscitation.
Insulin Replacement
The most significant change in the new guidance is the replacement of the traditional insulin sliding scale (variable rate intravenous insulin infusion (variable rate IVII)) with a fixed rate intravenous insulin infusion (fixed rate IVII). The predominant rationale for this change is the increasing prevalence of obesity, an insulin resistant state. Hence, the fixed rate is calculated per kilogram of body weight – initially 0.1 unit/kg/h. The previous recommendation for a ‘priming bolus’ has also been dropped, given that evidence has proven no benefit, providing the insulin infusion is started promptly, and in reality it was often omitted.
Continuation of a subcutaneous long-acting analogue insulin alongside the insulin infusion is now recommended (it facilitates a smoother transition back to the individual’s normal subcutaneous regimen, without rebound hyperglycaemia).
Metabolic Treatment Targets
- Reduction of blood ketone concentration by ≥ 0.5 mmol/l/h: generally measured hourly for the first 6 h; if ketonaemia is not responding adequately, reassess patient and increase fixed rate IVII by 1 unit/h, until adequate rate of resolution achieved.
- If blood ketone measurement is not available, aim to increase the venous bicarbonate by ≥ 3 mmol/l/h: again if there is an inadequate response to treatment, increase fixed rate IVII as above.
NB However, after 6 h, venous bicarbonate may become unreliable, particularly in the presence of hyperchloraemia as a consequence of the 0.9% sodium chloride infused.
- Alternatively, aim for a reduction in capillary blood glucose (CBG) of ≥ 3 mmol/l/h: again this is an alternative to ketone analysis; however, falling CBG is not an accurate indicator of the resolution of acidosis (especially in ‘euglycaemic acidosis’), which should be confirmed on venous gas analysis.
NB If ketones and glucose are not falling as expected, check that the insulin infusion pump is working and connected and that the expected amount of insulin has been infused.
- Maintenance of serum potassium between 4.0 and 5.0 mmol/l: serum potassium should be measured at least 2 hourly for the first 6 h, and at regular intervals thereafter.
Additional Measures
- Screen for infection: mid-stream urine sample, chest radiograph, blood cultures.
NB A moderate rise in CRP may be seen in DKA without intercurrent infection.
- ECG: screen for silent myocardial ischaemia, but also to assess the impact of hypo-/hyperkalaemia on the myocardium.
- Urethral catherisation: accurate fluid assessment is critical to effective management; placement of a urinary catheter is not mandatory, but if accurate urine volume assessment is not possible without a catheter, then one is required.
- A nasogastric tube should be placed if the conscious level is depressed, as acidosis predisposes to gastric stasis and the individual is therefore at high risk of aspiration.
- Adequate fluid and insulin therapy should produce prompt resolution of acidosis, and intravenous bicarbonate is therefore not routinely required and indeed may worsen the metabolic situtation – acidosis promotes a right shift in the oxygen dissociation curve which may be an adaptive response; rapid correction of acidosis with bicarbonate leads to a rise in arterial
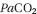 and a paradoxical fall in cerebrospinal fluid pH, exacerbating central nervous system depression.
and a paradoxical fall in cerebrospinal fluid pH, exacerbating central nervous system depression.
- Whilst it is recognised that whole body deficit of phosphate is high, routine phosphate supplementation has no proven benefit. However, in the case of respiratory and muscular weakness it may be considered.
Resolution of DKA is defined as a blood ketone level of < 0.3 mmol/l and venous pH > 7.3. If the individual is tolerating normal dietary intake they should be converted back to an appropriate subcutaneous insulin regimen. Trace/mild ketonuria may persist for a short time post-resolution of the acidosis.
The diabetes specialist team should be involved in the care of all DKA episodes, in particular the diabetes specialist nurse who will be required to check education/understanding of possible DKA precipitants and the required actions to deal with these, as well as to facilitate follow-up post-discharge.
Hyperosmolar Non-Ketotic Coma (HONK)
This typically occurs in the elderly, often previously undiagnosed, patient with type 2 DM. The onset may be drawn out, with polyuria precipitating dehydration over several days or even weeks. Precipitating factors include myocardial infarction, stroke and intercurrent infection. Unlike DKA, there is sufficient residual endogenous insulin to prevent ketogenesis, and hence no acidosis. Hyperosmolality is the predominant biochemical feature, in association with marked hyperglycaemia (blood glucose can exceed 100 mmol/l in extreme cases).
These patients are profoundly dehydrated and are at high risk of thrombosis (both arterial and venous), which is a significant contributor to the high mortality of this condition (30–50%). Low molecular weight heparin is therefore recommended unless there is a contraindication to its use (e.g. haemorrhagic stroke). Patients require rehydration to replace the massive fluid deficit, but this must be undertaken in a controlled manner (often with the aid of central venous pressure monitoring) especially where there is significant co-morbidity (e.g. cardiac disease). In addition, overzealous rehydration may cause rapid osmotic shifts, precipitating cerebral oedema. Paradoxically these patients are often very insulin sensitive and may need only small amounts of insulin to lower their blood glucose satisfactorily – aim for a fall of 3 mmol/l/h.
As with DKA, close monitoring of cardiovascular, renal and metabolic status, with regular checking of electrolytes, glucose, and osmolality is mandatory. Unlike DKA, insulin therapy is not always subsequently required once the acute episode has subsided, and survivors may manage on diet and oral hypoglycaemic agents.
Long-Term Diabetic Complications
Long-term complications occur in all forms of DM and are broadly classified as microvascular or macrovascular in origin (Table 17.8).
Table 17.8 Long-term Complications of Diabetes Mellitus
| Microvascular complications | Macrovascular complications |
| Retinopathy | Coronary heart disease |
| Neuropathy – peripheral and autonomic | Peripheral vascular disease |
| Nephropathy | Cerebrovascular disease (stroke, transient ischaemic attack) |
Macrovascular Complications
Diabetes is a major risk factor for the development of atherosclerotic vascular disease.
Attention to other Cardiovascular Risk Factors in the Patient with Diabetes Mellitus
Hypertension
With time, blood pressure targets in the context of diabetes have progressively fallen as data have emerged to show that in most cases ‘the lower the better’. Combination therapy is often required to achieve a blood pressure of ≤ 130/80 mmHg, or even lower in those with concomitant renal involvement. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are usually the preferred first-line agents, especially when there is evidence of microalbuminuria (see below). Calcium antagonists, α-adrenoceptor blockers and diuretics are useful adjuncts.
Dyslipidaemia
Several large-scale trials have shown that statins reduce both non-fatal and fatal cardiovascular events in patients with DM, when used in both primary and secondary prevention settings. Targets continue to be refined, but in general a total cholesterol level < 4 mmol/l with low density lipoprotein cholesterol (LDL-C) < 2 mmol/l are considered desirable.
Microvascular Complications
Retinopathy
- The most common cause of visual loss in adults of working age in the UK.
- Proliferative retinopathy is more common in type 1 DM, and maculopathy in type 2 DM.
- Good glycaemic control is the key factor in preventing the development of, or deterioration in, retinopathy.
- Other risk factors include hypertension, smoking and pregnancy.
Various classification schemes for diabetic retinopathy have been proposed based largely on ophthalmological findings or functional severity: Table 17.9 provides an example of a traditional classification scheme; however, others argue for a simplification to just ‘non-proliferative’ and ‘proliferative’ subgroups, but again with maculopathy considered as a separate entity.
Table 17.9 Classification of Diabetic Eye Disease
| Type | Features |
| Background | Microaneurysms |
| Haemorrhages (dot and blot/flame-shaped) | |
| Hard exudates (lipid deposits) | |
| Occasional (< 5) soft exudates (‘cotton wool’ spots) | |
| Preproliferative | As above |
| Soft exudates* | |
| Intraretinal microvascular abnormalities (IRMAs) | |
| Venous beading, venous reduplication | |
| Proliferative | New vessel formation at the disc/within one disc diameter (NVD) |
| New vessels elsewhere (NVE) | |
| Rubeosis iridis | |
| Maculopathy | Focal or diffuse oedema at the macula |
| Haemorrhages, exudates or other changes at the macula | |
| Advanced eye disease | Preretinal or vitreous haemorrhage |
| Retinal detachment | |
| Cataract | |
| * Multiple cotton wool spots signify retinal ischaemia. | |
Management
Modification of all treatable risk factors and annual screening (visual acuities and retinal photography) are the mainstay of prevention and early intervention. If background retinopathy is present, glycaemic control should be reviewed, microalbuminuria, hypertension and dyslipidaemia sought and actively treated, and the patient kept under close surveillance. The presence of preproliferative retinopathy requires prompt referral for formal ophthalmological review. Laser photocoagulation, in which laser therapy is applied to peripheral areas of the retina (thereby reducing total oxygen requirements across the retina, and ameliorating ischaemia in other areas that are critical for vision), may be indicated. Again, maintenance of good glycaemic, blood pressure and lipid control is of paramount importance. For proliferative retinopathy, laser photocoagulation can reduce visual loss: here laser therapy is used to induce regression of new blood vessels (thus reducing the risk of haemorrhage), in addition to reducing oxygen requirements throughout the retina, thereby retarding further new vessel proliferation. Laser therapy can also improve maculopathy. Vitrectomy (surgical removal of the vitreous) may be used as a salvage procedure in those with vitreous haemorrhage. It aims to improve vision by removing any blood from in or behind the vitreous, reattaches detached areas of retina, and is combined with pan-retinal laser photocoagulation to reduce the stimulus for further neovascularisation.
The potential role of antivascular endothelial growth factor treatments in the management of diabetic retinopathy is an area of current research interest, with early trials showing promising results. However, treatment programmes are likely to be costly and time-consuming.
Cataracts are more common and occur at an earlier age in patients with DM, and should therefore be actively sought and treated.
Microalbuminuria and Diabetic Nephropathy
- Data from the UK Renal Registry show that diabetic nephropathy is the most common single cause of end-stage renal failure amongst adults starting renal replacement therapy.
- Nephropathy, i.e. the development of macroalbuminuria and a progressive decline in renal function (with falling glomerular filtration rate (GFR) and rising serum creatinine), ultimately affects approximately 20–30% of patients with DM, although this percentage is falling as earlier and more aggressive management of DM and its complications is pursued.
- Microalbuminuria heralds the onset of diabetic renal disease and, if left unchecked, progresses to intermittent and then persistent proteinuria. Progression is associated with either localised (Kimmelstiel–Wilson nodules) or diffuse fibrotic thickening of afferent and efferent arterioles. Proteinuria, hypertension and oedema become clinically apparent. Serum creatinine only rises after significant renal damage has occurred.
- Incipient nephropathy is synonymous with microalbuminuria. Normal urinary albumin excretion is < 20 mg/l. Microalbuminuria is present when the urine albumin concentration is in the range 20–200 mg/l (30–300 mg/24 h), and macroalbuminuria when levels exceed this. Nephrotic syndrome is associated with urinary protein loss of > 3 g/24 h.
- Screening for microalbuminuria is most commonly undertaken through estimation of the urine albumin : creatinine ratio (ACR) on a spot urine sample : values > 2.5 mg/mmol in men and > 3.5 mg/mmol in women signify the presence of microalbuminuria if confirmed on at least one further independent sample during the next few months. In practice, 24-hour urine collections are now rarely performed.
- The presence of microalbuminuria is also indicative of progressive generalised vascular disease, and attention must be paid to the management of other cardiovascular risk factors (e.g. hypertension, dyslipidaemia, smoking history).
- Other causes of renal impairment may coexist (e.g. hypertension, renal artery stenosis) and should be sought if there are specific clinical pointers or atypical features (e.g. development of renal disease in the absence of retinopathy).
NB Intravenous contrast studies can precipitate acute renal failure in those with pre-existing renal impairment – adequate hydration and temporary withdrawal of agents such as metformin and ACE inhibitors/ARBs help reduce the risk in susceptible individuals – if in doubt, discuss with radiology and/or nephrology.
Management
The attainment of good glycaemic and, in particular, good blood pressure control is essential for the prevention and treatment of microalbuminuria. There is a large amount of data showing that ACE inhibitors are particularly effective in this setting, with ARBs a reasonable alternative in patients intolerant of the former. Both classes of agent appear to reduce proteinuria at levels above and beyond their antihypertensive effects.
Good blood pressure control remains the mainstay of treatment in those with established nephropathy, and again ACE inhibitors/ARBs are the preferred option(s), although additional antihypertensive therapy is often required, and diuretics may be helpful in treating fluid overload. Antihyperglycaemic agents that are long-acting and/or renally excreted (including insulin) should be reviewed on a regular basis and dosages adjusted or alternatives substituted where necessary.
NB Metformin should be avoided in those with established renal impairment (serum creatinine > 150 mmol/l or GFR < 30 ml/min) because of the risk of inducing lactic acidosis.
Early referral to a renal specialist service is generally recommended to ensure appropriate management of complications (e.g. secondary hyperparathyroidism, anaemia) and for consideration of/preparation for renal replacement therapy (dialysis and/or transplantation).
Continued aggressive management of cardiovascular risk factors is mandatory, as this is the major cause of morbidity and mortality in this group of patients.
Neuropathy
- More common in patients with a long history of DM (males > females), especially in those with poor glycaemic control.
- Multifactorial, reflecting a combination of metabolic and vascular factors.
Distal Symmetrical Polyneuropathy (‘Sensory Peripheral Neuropathy’)
This is the most common form of diabetic neuropathy (characterised by loss of both myelinated and unmyelinated nerve fibres) and typically affects the longest fibres first – hence the so-called ‘glove and stocking’ distribution. Symptoms include dysaesthesia (numbness and tingling), often worse at night, progressing to chronic pain (stabbing/burning/shooting) with time. Examination reveals absent ankle jerks, diminished vibration sense in the lower limbs, and an inability to feel the standard 10-g monofilament. Reduced pain and temperature sensation follow, with muscular wasting and weakness late features. Affected individuals are at high risk of developing diabetic foot complications (neuropathic/neuroischaemic ulcers, Charcot arthropathy).
Optimal diabetic control is central to the prevention of distal symmetrical polyneuropathy. Tricyclic antidepressants (e.g. amitriptyline), duloxetine (a serotonin and noradrenaline reuptake inhibitor) and various anticonvulsants (e.g. carbamazepine, gabapentin and pregabalin) may help with symptom relief. Topical capsaicin may also be tried.
Acute (Painful) Sensory Neuropathy
This is predominantly seen in male patients and is associated with poor glycaemic control or rapid improvement in glycaemic control (e.g. following commencement of insulin therapy).
Acute Mononeuropathies
These motor neuropathies are thought to result from ischaemia and occlusion of vasa nervorum. Various cranial nerves (e.g. third, fourth and sixth), the ulnar nerves and the lateral common peroneal nerves are most commonly affected, and more than one nerve can be involved at any given time (i.e. mononeuritis multiplex). It is often transient and spontaneous recovery of function usually occurs over a period of months, but can be variable.
Diabetic Amyotrophy (Proximal motor Neuropathy)
This is a rare disorder, which usually occurs in middle-aged men who develop painful, asymmetric weakness and wasting of the quadriceps muscles, and is often associated with marked anorexia and weight loss. Insulin, even in those with apparently satisfactory glycaemic control, remains the mainstay of treatment.
Autonomic Neuropathy
Autonomic neuropathy may manifest in a number of different ways, including:
- cardiovascular: postural hypotension, tachycardia, painless ischaemia
- gastrointestinal: constipation, diarrhoea (especially nocturnal), gastroparesis
- genitourinary: erectile dysfunction/impotence, atonic bladder
- others: hypoglycaemia unawareness, gustatory sweating, oedema.
Other Complications
The Diabetic Foot
Foot problems are very common and give rise to significant morbidity and mortality. The most commonly encountered problems are:
- Neuropathic ulceration – typically painless and occurring at pressure points (e.g. under the first and fifth metatarsal heads); lack of pain sensation and build-up of callus are predisposing factors; may be complicated by abscess formation/osteomyelitis. Ulcers should be swabbed and plain radiographs performed; if there is any suggestion of more deep-seated infection/osteomyelitis, consider MRI scan of the foot and isotope bone scintigraphy. Depending on the absence or presence of infection and the extent of tissue involvement, treatment may involve removal of callus and debridement, oral or intravenous antimicrobials and surgical drainage/debridement/amputation. Specialist non-weight-bearing footwear is also often required to facilitate healing.
- Neuroischaemic ulceration – here the situation is exacerbated by concomitant vascular insufficiency; patients may also complain of intermittent claudication and/or rest pain; ulcers also occur over the heel and dorsum of the foot/toes, and pre-gangrenous or frankly gangrenous changes may be present. Investigation and management is similar to that for neuropathic ulceration, but in addition the extent of vascular disease requires formal assessment, often with a combination of doppler ultrasound studies and arteriography to determine the feasibility of attempted revascularisation.
- Neuropathic joints (Charcot arthropathy) – a less common but well-recognised complication, often affecting the tarsometatarsal joints (i.e. mid-foot) and/or the ankle; impaired sensation with abnormal mechanical stresses/load-bearing may contribute to its development/progression. Left unchecked, joint instability and trophic bone changes lead to major and severe deformities of the foot. MRI scanning can help to differentiate from infection and, in combination with isotope bone scintigraphy, may reveal new bone formation. If recognised at an early stage, intravenous bisphosphonates may help in preventing bone resorption; however, the mainstay of treatment is to prevent deformity and destruction of the foot by strict avoidance of weight bearing, e.g. through the use of a total contact cast. Surgical reconstruction of affected joints may be indicated in refractory cases.
Diabetes-Related Skin Conditions
Skin changes in diabetes may reflect:
- an immediate consequence of hyperglycaemia, e.g. opportunistic infection including candidiasis (particularly genital, e.g. balanitis) and S. aureus folliculitis
- long-term changes resultant from chronic hyperglycaemia
- a consequence of treatment.
Necrobiosis lipoidica diabeticorum (pretibial diabetic dermopathy) is pathognomonic of DM. It is characterised by atrophy of subcutaneous collagen, usually over the shins. Lesions typically start as small, brownish, erythematous shiny patches and may evolve to develop a central yellow area with atrophy that can ulcerate. Unfortunately, no treatment has been shown to be of benefit – the most important management is to protect the lesions from trauma.
Granuloma annulare typically manifests as a cluster of small papules that form a ring on the back of the hands or feet. Although spontaneous resolution usually occurs, cryotherapy or intralesional corticosteroid therapy may hasten the process.
Skin changes associated with insulin resistance include acanthosis nigricans (pigmented velvety thickening of the skin, typically seen in the axillae and nape of the neck, and considered a marker of severe insulin resistance) and scleroderma diabeticorum (thickening of the skin on the upper back and neck).
Lipoatrophy is painless localised necrosis of subcutaneous fat tissue at the site of insulin injection therapy; it is now uncommon following the introduction of recombinant/analogue human insulins. In contrast, lipohypertrophy (localised accumulation of fat tissue at the site of multiple injections) is very common, as hypertrophied sites are relatively painless to inject into! However, the insulin absorption from these sites is erratic, leading to unpredictable glycaemic excursions.
Diabetes and Pregnancy
Pregestational DM
Commissioned in 2002, and reporting in 2007, the UK Confidential Enquiry into Maternal and Child Health (CEMACH) quantified the risks of diabetic pregnancy: perinatal mortality was 3–5 times higher and congenital malformation rate 4–10 times higher than in the general population; ‘suboptimal care’ was highlighted as a major factor in those cases with poor outcomes.
Prepregnancy Counselling
The importance of optimal diabetic control before conception should be strongly emphasised. Women should be given realistic information regarding the effects of diabetes on pregnancy (miscarriage, congenital malformation, stillbirth and neonatal death) and advised that these risks can be reduced (but not eliminated) with good preconceptual and antenatal glycaemic control. Counselling should also discuss the potential effects of pregnancy on the progression of diabetic complications.
Antenatal Care
Daily oral folic acid supplements (5 mg) should be taken from 3 months prior to conception, and for at least the first 12 weeks of gestation, to reduce the risk of neural tube defects. Tight glycaemic control must be maintained throughout pregnancy. To facilitate this, women should undergo an early antenatal assessment (as soon as pregnancy is confirmed) and antenatal care should be provided by a multidisciplinary team (as described above).
Glycaemic Control
Targets for self-monitoring of blood glucose (HBGM) should be agreed with the individual woman. Where possible, aim to keep fasting blood glucose levels between 3.5 and 5.9 mmol/l. HBGM should be performed 1h after each meal, with the aim, where safe, of keeping levels below 7.8 mmol/l. HBGM should also be performed before retiring to bed each night.
NB HbA1c is less reliable in the second and third trimester to gauge the adequacy of control.
Women should have regular contact with the diabetes centre, ideally on a weekly basis (and no less than fortnightly), for regular reassessment. They must be advised of the increased risk of hypoglycaemia in pregnancy, and that hypoglycaemia unawareness is also more common – this is particularly important with respect to driving.
It is also important to warn of the increased risk of ketogenesis and the particular hazards to the fetus/pregnancy of ketosis. Clear guidance should be offered regarding testing for ketosis and the ‘sick day rules’ (see below) reiterated. Ketone testing strips (either for ketonuria or ketonaemia) should be prescribed.
Medication
All oral hypoglycaemics with the exception of metformin, should be discontinued prior to conception or as soon as pregnancy is confirmed. Metformin can be offered as a first-line agent where glycaemic control has not been achieved through lifestyle measures alone.
NB Although the use of metformin is supported by NICE, it is not currently licensed in pregnancy and women should therefore be appropriately counselled.
If glycaemic control is not achieved rapidly, i.e. within 10–14 days, insulin therapy should be commenced. An MDI (‘basal-bolus’) regimen is preferred for all women requiring insulin therapy. Although NICE does not currently recommend the routine use of basal analogue insulin preparations in pregnancy, most units will continue their use if started preconceptually, and indeed many diabetologists would also initiate treatment with them when required, because of their favourable hypoglycaemic profile.
Drugs affecting the renin-angiotensin system (e.g. ACE inhibitors, ARBs) should be stopped as soon as possible as they are potentially fetotoxic. Alternative antihypertensive agents (e.g. methyldopa, labetalol) can be substituted where necessary.
Where an ACE inhibitor/ARB has been used prior to pregnancy for the purpose of renoprotection, close surveillance for nephropathy must be observed. Statins should be discontinued, and the risk : benefit ratios of all other medications must be carefully discussed.
Retinal Assessment
Women should be advised of the importance of retinal assessment in the preconception period, and during and after pregnancy.
Renal Assessment
Renal assessment is important both in the preconception period and during pregnancy. Diabetic nephropathy is a progressive disease that can significantly adversely affect the outcome of the pregnancy; in addition, pregnancy can accelerate the progression of nephropathy. Early involvement of the renal team is therefore advisable in any patient in whom there is concern regarding renal status.
NB eGFR is not validated for use in pregnancy and should not therefore be used as an indicator of renal function.
Fetal Growth and Well-being
Women should have all aspects of routine fetal monitoring and well-being offered. In addition a four-chamber view of the fetal heart and outflow tracts should be offered at 18–20 weeks’ gestation. Assessment of fetal growth and amniotic fluid volume by ultrasonography every 2–4 weeks’ is recommended between 28 and 38 weeks’ gestation. Routine monitoring of fetal well-being (e.g. cardiotocography) prior to 38 weeks is not generally recommended; however, in women at risk of intrauterine growth retardation (IUGR; i.e. those with macrovascular disease/nephropathy) an individualised approach should be tailored to assess fetal growth and well-being.
Women with DM in pregnancy should be advised of the possibility of fetal macrosomia and its associated risks (e.g. birth trauma, and an increased requirement for induction of labour and/or caesarean section).
Intrapartum Care
Delivery should be completed by 39 weeks’ gestation; every woman should have an individualised care plan for her glycaemic control during the intrapartum and immediate postpartum period, and this should be clearly documented in her notes. During labour, blood glucose must be monitored hourly, aiming to keep levels between 4 and 7 mmol/l; if this cannot be achieved using the patient’s standard diabetes treatment, then a variable rate intravenous insulin infusion (i.e. sliding scale) should be initiated.
Neonatal Care
Blood glucose testing is carried out routinely in all offspring of mothers with DM, and babies should remain in hospital for 24 h post-delivery to ensure maintenance of adequate glucose levels.
Preterm Labour
In the case of threatened or actual preterm labour, women with DM receiving corticosteroids antenatally require admission for blood glucose monitoring. If glycaemic control is not maintained between 4 and 7 mmol/l on the patient’s regular treatment regimen, then a variable rate intravenous insulin infusion should be initiated and continued for 24 h after the last dose of steroids.
Gestational Diabetes Mellitus
Gestational diabetes mellitus (GDM) is diabetes that develops or is first recognised in pregnancy. In the UK, the prevalence varies from 1 in 220 to 1 in 330 depending on the ethnicity of the region. Both IADPSG and NICE have recently issued guidance on the definition of, and screening for, GDM (see classification, above).
Management is along similar lines as for pregestational DM, with a particular emphasis on diet and HBGM; target glycaemic levels and fetal surveillance strategies are the same. Glucose tolerance often returns to normal after delivery. NICE recommends that a fasting venous plasma glucose level be checked at 5–6 weeks postnatally, repeated on an annual basis thereafter, as it is well recognised that a substantial proportion of women affected with GDM go on to develop type 2 DM in later life, with estimates varying from 20–50% depending on the population under study.
Other Important Information for Patients with Diabetes
Diabetes and Intercurrent Illness
‘Sick Day Rules’
During times of illness, particularly when oral intake is poor, many insulin-dependent/treated patients with DM become concerned regarding the risk of developing hypoglycaemia; however, in reality hepatic glycogenolysis provides sufficient glucose to prevent the development of hypoglycaemia during most intercurrent illnesses, even when calorie intake is markedly reduced. In fact, the main risk with intercurrent illness is of developing hyperglycaemia, precipitated by relative insulin resistance, which may lead to DKA/HONK, particularly if insulin doses are inappropriately omitted or reduced. It is important therefore that patients are educated to:
- never stop insulin during intercurrent illness
- perform HBGM frequently
- check for ketones regularly
- maintain a high fluid intake wherever possible.
Most patients require frequent small doses of rapid-acting insulin to avoid developing hyperglycaemia and, in the case of type 1 DM, ketoacidosis. If in doubt, they should be advised to seek early medical advice. This information should be provided in written format, as well as verbally, and reinforced at each annual review.
Diabetes and Surgery
Most hospitals have protocols for the management of individuals with DM undergoing procedures that require a period of fasting, e.g. colonoscopy through to major abdominal surgery. Ideally patients should be given written as well as verbal instructions on medication adjustments at their preprocedural/preoperative assessement. To facilitate optimal glycaemic control, those with DM should be scheduled as early on the operative/procedure list as is practical, and a variable rate intravenous insulin infusion instituted to maintain euglycaemia as the stress of surgery (cortisol, glucagon, growth hormone and catecholamine surges) predispose to hyperglycaemia. The variable rate intravenous insulin infusion is usually combined with an infusion of 5% dextrose containing 20 mmol/l potassium chloride, but tailored to the individual’s medical/electrolyte status. Once the patient is eating and drinking normally postoperatively, it is usually possible to return to his/her regular therapy.
Diabetes and Driving in the UK
In the UK, patients with DM treated with tablets and/or diet may hold a ‘car/motorcycle’ group 1 licence and need not inform the DVLA unless they have complications (see below for specific rules relating to hypoglycaemia); however, they must inform their insurance company. The DVLA (and insurance company) must be notified once a patient commences insulin therapy. Those treated with insulin will be given a group 1 entitlement licence for 1, 2 or 3 years depending upon the quality of their glycaemic control, the regularity of medical surveillance and the extent of complications. Patients with type 2 DM not requiring insulin may be issued with a ‘till 70’ licence, unless they have complications of their DM. Recent changes to DVLA regulations mean that it is now possible for a patient receiving insulin to hold a group 2 (LGV, PCV) licence with review on an annual basis; however, a series of strict medical criteria must be met (in particular with respect to hypoglycaemia (preserved awareness, regular recorded testing, no episodes requiring third party rescue, etc.), absence of complications, and annual review by an independent Consultant Diabetologist).
Hypoglycaemia is the major factor that impacts on driving in all categories. Patients must be able to effectively recognise and treat hypoglycaemia. The DVLA advises that patients should check their blood glucose at times relevant to driving. Patients must carry glucose tablets (or equivalent) to treat hypoglycaemia, as well as their glucometer; they are expected to stop and test their glucose level every 2 h on a long journey. If a hypoglycaemic episode occurs whilst driving, they should pull over, remove the keys from the ignition and move to the passenger side, so they are no longer in charge of the vehicle. They must wait an appropriate time after correcting the hypoglycaemia before recommencing their journey. Hypoglycaemia requiring third party rescue is considered a major concern and will lead to even a group 1 licence being revoked if it has occurred on more than one occasion in the preceding 12 months.
Dyslipidaemia
The major importance of dyslipidaemia lies in its close relationship with cardiovascular disease. At a simplistic level, LDL cholesterol is potentially ‘deleterious’, as small dense LDL particles are capable of penetrating the endothelial barrier and are taken up by macrophages before undergoing oxidation to give rise to ‘foam cells’. The latter contribute to atheromatous plaque formation and instability, e.g. in coronary blood vessels. In contrast, HDL particles predominantly work to remove excess cholesterol from cells for transport back to the liver in a process known as reverse cholesterol transport. Hence, LDL cholesterol is sometimes referred to as ‘bad cholesterol’ and HDL as ‘good cholesterol’. Hypertriglyceridaemia is also considered an independent risk factor for vascular disease, and predisposes to pancreatitis.
Classification
A practical classification is shown in Table 17.10.
Table 17.10 Classification of Dyslipidaemia
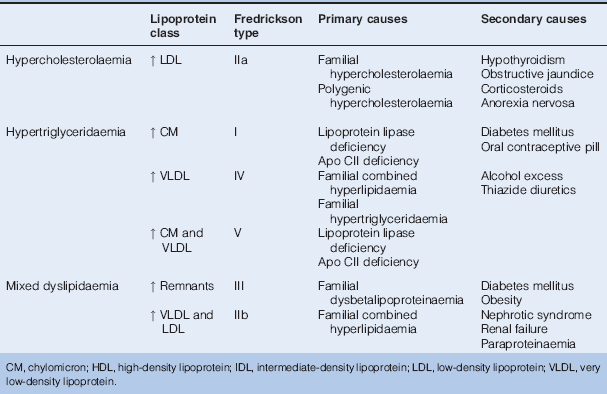
Primary Dyslipidaemias
Familial Hypercholesterolaemia
Familial hypercholesterolaemia (autosomal dominant) is caused by mutations in the LDL receptor gene, which results in reduced LDL cholesterol clearance and consequent hypercholesterolaemia (severe in the homozygous form, less marked in heterozygotes) leading to premature atherosclerosis. Affected individuals are often asymptomatic but manifest clinical signs including corneal arcus, xanthelasmata and tendon xanthomata. Left untreated, premature cardiovascular death is common. Heterozygotes respond to pharmacological management of their hypercholesterolaemia and modification of other cardiovascular risk factors. Homozygotes often fair badly and may require liver transplantation. Family screening is strongly recommended.
Familial Defective Apolipoprotein B-100
This is caused by a single amino-acid substitution in apolipoprotein B, resulting in defective binding of LDL to its receptor. It is clinically indistinguishable from familial hypercholesterolaemia and treated in the same way.
Polygenic Hypercholesterolaemia
Often considered a diagnosis of exclusion in which there is isolated LDL cholesterol elevation without peripheral stigmata, but premature ischaemic heart disease within a family. Variants in Apo E4 alleles have been implicated in some instances. Statins are the preferred treatment option.
Familial Hypertriglyceridaemia
This may be caused by either increased hepatic VLDL production or a failure of triglycerides to be cleared from chylomicrons by lipoprotein lipase. Inheritance is autosomal dominant. Marked hypertriglyceridaemia is associated with eruptive xanthomata, lipaemia retinalis and acute pancreatitis. Management involves avoiding/treating potential exacerbating conditions such as obesity, hypothyroidism and diabetes mellitus, together with pharmacotherapy aimed at reducing the hypertriglyceridaemia (e.g. fibrates and nicotinic acid).
Familial Combined Hyperlipidaemia
This condition is inherited as an autosomal dominant trait and affects ~1% of the general population but up to 15% of patients suffering myocardial infarction who present before 60 years of age. It is characterised by an overproduction of hepatic-derived Apo B100 (genetic basis unknown). Cholesterol and triglycerides are classically both increased (with increases in LDL and/or VLDL cholesterol; HDL cholesterol is typically low), but one or other may be normal. Clinical signs include corneal arcus and xanthelasmata, but not tendon xanthomata. The risk of atherosclerosis is increased. Treatment often requires combination statin (targeting LDL cholesterol) and fibrate (triglyceride lowering) therapy, with the attendant increased risks of rhabdomyolysis and hepatic upset.
Familial Dysbetalipoproteinaemia
This is a rare (autosomal recessive) disorder characterised by elevations in triglyceride and total cholesterol levels. The disease develops in individuals who are homozygous for apolipoprotein E2 (Apo E2) variants. There is a reduced ability to convert VLDL and IDL to LDL particles in the blood and decreased clearance of chylomicron remnants. Affected individuals are at increased risk of atherosclerotic cardiovascular disease and peripheral vascular disease. Linear xanthomata of the palmar creases are considered pathognomonic. The condition responds well to avoidance/treatment of other disorders that predispose to hypertriglyceridaemia, and to medications that reduce blood triglyceride concentrations (e.g. fibrates, nicotinic acid).
Secondary Dyslipidaemias
Secondary dyslipidaemias are very common, and treatment of the underlying cause, with the exception of gout and chronic renal failure, generally improves the dyslipidaemia. Common secondary causes include endocrine disorders (e.g. hypothyroidism, Cushing syndrome, acromegaly), renal failure/nephrotic syndrome, drugs (e.g. oral oestrogen, thiazide diuretic or retinoid therapy), alcohol excess, obstructive liver disease, diabetes mellitus and obesity.
Associations Between Lipids and Vascular Disease
Epidemiological studies such as the Multiple Risk Factor Intervention Trial (MRFIT) and Prospective Cardiovascular Munster study (PROCAM) have examined the potential links between dyslipidaemia and ischaemic heart disease. In general, most have shown a strong curvilinear association between plasma total cholesterol or LDL cholesterol and ischaemic heart disease (IHD), whereas there is an inverse relationship with HDL cholesterol. For example, a 10% increase in total/LDL cholesterol increases the IHD risk by 20%. (As 60–70% of plasma cholesterol is present in LDL, total cholesterol is often used as a surrogate measure of LDL cholesterol.) Raised levels of lipoprotein A also predict the risk of ischaemic heart disease. In contrast, studies have given variable results with regard to the association of hypertriglyceridaemia and IHD, although on balance it appears that raised plasma triglyceride levels are a risk factor.
Clinical Presentation
Dyslipidaemia is typically identified in the context of cardiovascular disorders or other related metabolic conditions (e.g. diabetes mellitus). Occasionally the clinical stigmata of dyslipidaemia trigger screening (xanthelasmata, corneal arcus and tendon xanthomata are associated with hypercholesterolaemia, and eruptive or tuberous xanthomata and lipaemia retinalis with hypertriglyceridaemia). Screening should also be offered to asymptomatic individuals with a positive family history. Patients with more marked hypertriglyceridaemia (> 10 mmol/l) are prone to pancreatitis.
Investigation
Total Cholesterol
Fasting samples are not strictly necessary, although should be requested when patients record non-fasting cholesterol levels that would require treatment, and in those suspected of having dyslipidaemia.
NB A mild or moderate elevation in LDL cholesterol with an associated reduction in HDL cholesterol can result in a normal total cholesterol level, which can therefore be misleading with respect to cardiovascular risk.
LDL Cholesterol
A fasting sample is required for an accurate result. However, the assay is difficult and expensive to run and the majority of laboratories actually calculate/estimate the LDL level, from the Friedewald equation (LDL cholesterol = (Total cholesterol) − (HDL cholesterol) − (triglycerides/2.2)) (all values in mmol/l).
NB The Friedewald equation should not be used when chylomicrons are present, when plasma triglycerides exceed 4.5 mmol/l or in patients with known dysbetalipoproteinaemia.
HDL Cholesterol
Measurement is not standardised and there are generally only small differences between normal and abnormal levels. However, the ratio of total serum cholesterol : HDL-cholesterol is commonly used in coronary risk prediction charts.
Triglycerides
Plasma triglycerides rise dramatically after a meal so a fasting sample, preferably overnight, is required.
Other Investigations
- Fasting blood glucose: to exclude dyslipidaemia secondary to DM.
- Renal function: to exclude chronic kidney disease.
- Liver function tests (transaminases): to exclude intrinsic liver disease, and as a baseline for monitoring while on statin therapy.
- Creatine kinase: some advocate measuring at baseline prior to statin therapy, while others suggest checking only in those complaining of muscle symptoms while on treatment.
- Thyroid function tests: to exclude hypothyroidism.
- Genetic testing/screening: as clinically indicated (see above).
Management
The primary purpose of treating dyslipidaemia is to prevent or reduce the risk and complications of cardiovascular disease. There is a large volume of powerful clinical trial data to show that lipid-lowering therapy reduces the risk of cardiovascular morbidity and mortality in at-risk individuals – both in the context of primary and secondary prevention. The decision to initiate treatment in those without a prior history of vascular disease is often based on an assessment of overall risk of coronary heart disease. For example, this can be calculated from cholesterol levels, age, sex and blood pressure using risk tables, such as those provided by the Joint British Societies (available at http://www.bhsoc.org/Cardiovascular_Risk_Charts_and_Calculators.stm).
Current Joint British Societies’ guidelines recommend that in those with:
- established IHD
- diabetes mellitus and
- in asymptomatic individuals at high cardiovascular risk (i.e. 10-year IHD risk > 20%)
lipid targets should be:
- total cholesterol < 4.0 mmol/l and LDL-cholesterol < 2.0 mmol/l
or
- a 25% reduction in total cholesterol and a 30% reduction in LDL-cholesterol, whichever achieves the lowest absolute values.
However, NICE considers that the case for the cost- effectiveness (including adverse events) of higher intensity statin therapy (either alone or in combination with other agents) to reduce CVD events by treating to target levels of total cholesterol of either 5 mmol/l or 4 mmol/l (or comparable LDL-cholesterol levels) has yet to be proven.
Non-Drug Therapy
Diet
Dietary modification may lower cholesterol and triglyceride levels, but typically only by 5–10% (remember, < 15% of cholesterol is dietary in origin). Longer chain saturated fatty acids raise and mono- and polyunsaturated fatty acids lower LDL cholesterol.
In general, individuals should aim for:
- total fat intake ≤ 30% of total energy intake
- saturated fats ≤ 10% of total energy intake
- dietary cholesterol < 300 mg/day
- replacement of saturated fats with mono- or polyunsaturated fats
- five portions of fruit and vegetables per day
- two portions of fish per week, including one portion of oily fish.
Other Lifestyle Measures
- weight reduction
- regular physical exercise
- smoking cessation
- avoidance of excess alcohol ingestion
- good blood pressure and glycaemic control
Drug Therapy
Statins
Statins are competitive inhibitors of HMG CoA reductase. They are potent in lowering LDL cholesterol, but less effective than fibrates in reducing triglycerides or raising HDL cholesterol levels. They reduce cardiovascular disease events irrespective of the starting cholesterol concentration, although patients with a total serum-cholesterol concentration of ≥ 5 mmol/l are likely to derive most benefit. In diabetes mellitus it is generally advised that all patients > 40 years of age be considered for statin therapy, which may also be indicated in younger subjects with complications (however, statins should be avoided in females desiring pregnancy owing to risk of fetal anomalies).
Myositis (which can lead to rhabdomyolysis) is a rare, but potentially serious, adverse effect of statin therapy – patients should be warned to promptly report unexplained muscle pain, tenderness or weakness. Liver function should be monitored during treatment.
NICE currently recommends simvastatin as first-line therapy, but allows for the use of other statins with similar acquisition costs. Other options include atorvastatin, fluvastatin, pravastatin and rosuvastatin.
Simvastatin is initiated at a dose of 40 mg/day (usually taken at night-time) unless potential drug interactions or adverse effects are a concern, when a lower dose of simvastatin or pravastatin should be offered. Dose reduction or temporary cessation of treatment may need to be considered in cases where there is a ‘temporary’ drug interaction or intercurrent illness that predisposes to statin toxicity.
Fibrates
Fibrates (which are high affinity ligands for the nuclear receptor peroxisome proliferator-activated receptor α (PPARα)) act mainly by decreasing serum triglycerides; they also tend to raise HDL cholesterol levels, but have variable effects on LDL cholesterol. They are generally considered first-line therapy only in those who have marked hypertriglyceridaemia (> 10 mmol/l) or in those who cannot tolerate a statin.
The fibrates may also cause a myositis-like syndrome (particularly in those with chronic renal impairment), and this risk is significantly increased when used in conjunction with statins. Dual therapy should therefore only be initiated under expert supervision.
Bile Acid Sequestrants
These agents bind bile acids in the small intestine, preventing their reabsorption, which in turn promotes hepatic conversion of cholesterol into bile acids; the resultant increase in hepatic LDL receptor expression/activity increases LDL cholesterol clearance from plasma. Colestyramine, colestipol and colesevelam are all effective in reducing hypercholesterolaemia, but may worsen hypertriglyceridaemia and cause malabsorption of fat-soluble vitamins.
Nicotinic Acid Group (Acipimox and Nicotinic Acid)
Agents in this category reduce the synthesis of both cholesterol and triglycerides; they also increase HDL cholesterol. However, their use is limited by side effects, particularly vasodilatation leading to flushing. Nicotinic acid is licensed for use with a statin or in those intolerant of statins.
Ezetimibe
Ezetimibe inhibits the intestinal absorption of cholesterol. It is licensed as an adjunct to dietary manipulation in patients with primary hypercholesterolaemia in combination with a statin (or alone if a statin is inappropriate or not tolerated), and in patients with homozygous familial hypercholesterolaemia in combination with a statin. Side effects include gastrointestinal upset, and there is an increased risk of myositis/rhabdomyolysis when used in conjunction with a statin.
Omega-3 Fatty Acid Compounds (Commonly Known as Fish Oils)
These may be used as an adjunct to reduce serum triglycerides; however, there is little evidence that they reduce cardiovascular risk.
Obesity
Traditionally, body mass index (BMI = weight in kilogrammes divided by height in metres squared) has been used to categorise body weight: < 18.5 = underweight; 18.5–24.9 = normal; > 25 = overweight; > 30 = obese; > 40 = severe/morbid obesity. However, BMI takes no account of body composition, overestimating obesity in muscular individuals and failing to discriminate between central (visceral) and peripheral (gluteal, limb) adipose tissue accumulation. The former (i.e. central obesity) is particularly associated with metabolic dysfunction (and the metabolic syndrome) and increased cardiovascular risk. In addition, obesity predisposes to sleep apnoea, joint failure (especially hips and knees) and cancer.
The prevalence of overweight/obesity has been estimated to be as high as 50% or more in Western societies, and childhood/adolescent obesity is an increasing problem. Sadly, the developing world is also catching up as the ‘obesity epidemic’ becomes truly global.
Aetiology
In most instances, obesity is the result of complex interactions between genetic, environmental and behavioural factors. Although a sedentary lifestyle and excess caloric intake are major predisposing factors, there is increasing evidence to suggest that not only extreme forms of obesity but also more common variants are linked to genetic predisposition, i.e. put simply, some individuals are genetically more prone to weight gain than others. Table 17.11 lists some of the recognised factors that contribute to being overweight/obese.
Table 17.11 Factors Implicated in Weight Gain/Obesity
| Type | Example(s) |
| Diet | Simple over-eating (especially energy dense foods) |
| Exercise | Sedentary work/lifestyle |
| Enforced inactivity (e.g. following surgery/injury) | |
| Ageing | |
| Social/behavioural | Habitual eating |
| Binge-eating | |
| Genetic variation | |
| • Common variants | FTO gene polymorphisms |
| • Rare conditions | Leptin and leptin receptor gene defects, melanocortin 4-receptor gene defects; Prader–Willi syndrome; Laurence–Moon–Biedl syndrome |
| Drugs | Glucocorticoids, oral contraceptive preparations, sulphonylureas, insulin, TCAs, SSRIs, olanzapine, clozapine, lithium, sodium valproate |
| Endocrine disorders | Hypothyroidism |
| Cushing syndrome | |
| Hypothalamic disorders | |
| Others | Low birth weight (‘fetal programming’) |
| Cardiac/renal/hepatic failure – with fluid retention | |
| FTO, fat mass and obesity associated gene; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants. | |
Clinical Features
Age at onset, previous success/failure in attempts to lose weight, family history, diet/alcohol consumption and physical activity should all be enquired about when assessing patients who are overweight/obese. Specific features of conditions that predispose to obesity (e.g. hypothyroidism, Cushing syndrome) should also be sought and a careful drug history taken. Clinical examination is important in determining the pattern of obesity (e.g. global versus central) and identifying specific markers of insulin resistance (e.g. acanthosis nigricans) or other disorders.
Investigation
If there is suspicion of an underlying predisposing condition (e.g. hypothyroidism, Cushing syndrome, monogenic form of obesity), then investigations should be tailored accordingly. In all other cases, investigations are directed towards assessment of cardiovascular risk and other comorbidities.
Management
Wherever possible, underlying predisposing disorders should be treated and offending drugs withdrawn/substituted. Care of the obese patient is best provided by a multidisciplinary team, which usually includes a physician trained in obesity medicine, a nurse specialist, obesity dietitian and a psychologist. The treatment strategy typically involves five elements:
- Dietary modification: a variety of regimes exist, which in general involve three phases: (1) a screening phase, (2) an intensive weight loss phase and (3) a weight maintenance phase. Specialist advice should be sought if considering the use of more calorie restricted regimes, to minimise the risk of adverse effects.
- Exercise programme: in particular, aerobic exercise.
- Behavioural modification: a core feature of any weight reduction programme; may involve goal setting, meal planning, self-monitoring and group work.
- Medical therapy: considered if dietary/behavioural modification/exercise in combination are ineffective. Currently, the gastric/pancreatic lipase inhibitor orlistat is the only agent licensed for use in the UK: it facilitates mild to moderate weight loss when used in combination with lifestyle measures, but may be associated with malabsorption of fat soluble vitamins. Although initially introduced to improve glycaemic control in type 2 DM, GLP-1 agonists/analogues (e.g. exenatide and liraglutide – see diabetes mellitus) have shown great potential to promote weight loss, and studies to assess their efficacy in this regard outside the context of DM are in progress.
- Bariatric surgery: two main forms exist – gastric banding or stapling and malabsorptive (i.e. bypassing the small bowel) – which are sometimes combined; both types are effective in promoting weight loss, but long-term outcomes/adverse effects are still unknown.
Prognosis
- Data from prospectively followed cohorts, such as the Framingham study, indicate that morbidity and mortality are substantially increased in obese individuals.
- Common complications of obesity include hypertension, diabetes mellitus, ischaemic heart disease, osteoarthritis/joint failure, herniae, gallstones and varicose veins. In women there is an increased incidence of hirsutism/menstrual disturbance and breast and endometrial carcinoma. Obese subjects also present an increased surgical risk.
Under(mal)nutrition
Undernutrition is a major problem of the developing world. Marasmus refers to severe protein-energy malnutrition. Children are grossly underweight with muscle wasting and markedly diminished fat. There is no oedema. In kwashiorkor there is protein deficiency, but with adequate calorie intake. There is oedema from hypoproteinaemia, and lipids accumulate in the liver, causing hepatomegaly.
In industrialised countries, undernutrition may be seen during any acute illness, but particularly those involving the gastrointestinal tract. Protein loss may also be substantial following burns (due to cutaneous loss), and can arise postoperatively (reflecting reduced intake and increased catabolism).
Vitamin Deficiencies
Vitamins are organic substances, each with specific biochemical functions. They are mostly found in food and typically only required in small amounts.
Fat-Soluble Vitamins
- Vitamin A (retinol): found in liver, fish and dairy products; it is also produced in the intestine by cleavage of β-carotene (found in carrots and other vegetables); it is required for night vision (11-cis-retinaldehyde combines with rhodopsin in retinal rods) and is important for epithelial keratinisation; deficiency is rarely seen in industrialised countries, but it is associated with night blindness, xerophthalmia (a relatively common cause of blindness in the developing world) and increased susceptibility to infections. Overdosage reduces keratinisation of skin and sebum production (causing rough, dry skin and hair, but explaining the efficacy of agents such as isotretinoin in the treatment of conditions such as acne) and liver enlargement. Vitamin A and its derivatives should be avoided in pregnancy (teratogenic).
- Vitamin D (see metabolic bone disorders).
- Vitamin E (α-tocopherol and related compounds) is found in vegetable oils. It is anti-oxidant and present in all cell membranes. Severe deficiency, especially in childhood, may predispose to haemolytic anaemia, and muscular and neurological disorders.
- Vitamin K is found in green vegetables. It is a cofactor for the synthesis of several clotting factors (II, VII, IX and X), and deficiency causes a prolonged prothrombin time and bleeding tendency. Oral coumarin anticoagulants (e.g. warfarin) act by interfering with vitamin K metabolism in hepatocytes.
Water-Soluble Vitamins
- Vitamin B1 (thiamine): found in many foods, including wheat, cereals and meat. Deficiency rapidly occurs if dietary intake is low as body stores are small. It is a cofactor in many metabolic pathways. Deficiency causes:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


