Chapter 26 Melanoma
Melanoma
Clinical features
Over the last several decades, the conspicuous increasing incidence of this malignant neoplasm has made this disease more prominent, linked to some rise in morbidity and mortality, although this appears now to be somewhat abating.1–7 At present it accounts for approximately 4% and 5% of all new malignancies in females and males, respectively.5 This has made melanoma the sixth and seventh most common cancers in the United States in terms of estimated new cases in 2010. However, the number of deaths from this disease places it well outside the top 10 causes of cancer-related deaths for both sexes at 8,700 annually. Mortality for this disease is still increasing in males, but has leveled off and appears to be decreasing in females, according to present data.6 Currently, the average rate of increasing incidence is of the order of 4–6% per year.7–9 The estimated number of new cases of melanoma per year is in excess of 68 000.6,10 Projections for individuals born in 2000 estimate that 1 person in 75 will ultimately develop the tumor.11
Although melanoma is seen predominantly in adults, it occasionally presents in children.12–16 The latter often have an associated risk factor, such as xeroderma pigmentosum, congenital bathing-trunk nevus, familial dysplastic nevus syndrome or familial melanoma and possibly immunosuppression. Pediatric melanoma accounts for 1–3% of all childhood malignancies.17 Its biological behavior is more variable and difficult to predict than melanoma in adults; however, metastasis rates of 39% and overall mortality of 29% after 5 years or more follow-up have been documented.16,18 Transplacental spread with resultant congenital melanoma has been documented exceptionally rarely.19
Precise figures are not available, but considering that the average adult has approximately 15–40 acquired melanocytic nevi and that the incidence of melanoma in the USA is roughly 30 per 100 000 of the population per annum, then the likelihood of any one benign acquired melanocytic nevus undergoing malignant transformation is remote.2,26 On the other hand, at least 35% of nodular and superficial spreading melanomas arise within a background of melanocytic nevi (congenital, acquired or dysplastic).3,20,27–29 Only very rarely do the more typical (small) congenital nevi develop melanomatous features. Giant ‘bathing-trunk’ variants, however, are a greater cause for concern, with approximately 3–18% undergoing malignant transformation.30,31 Lentigo maligna melanoma is not associated with pre-existent benign or dysplastic nevi.
While the etiology is multifactorial, including genetic and racial factors, by far the most important known predisposing environmental agent in the majority of tumors (with the exception of acral lentiginous and mucosal variants) is excessive exposure to ultraviolet (UV) light.32 Surprisingly, the contribution of the UV is modest, with an estimated 1.7-fold increase in risk.33 Although traditionally UVB has been regarded as the causative agent, more recent evidence implicates UVA, at least in some patients.34–36 Melanin pigment protects against the effects of solar irradiation and therefore cutaneous melanoma is rare in dark-skinned races, except for albinos and at those sites where pigment is absent (e.g., nail beds, palms, soles, and mucous membranes). UV irradiation, possibly by inducing free-radical formation, results in the development of dimers between adjacent pyrimidine molecules. Patients who lack the necessary endonucleases for repairing such damage, such as those with xeroderma pigmentosum, have a greatly increased risk of developing cutaneous neoplasms, including melanoma.
Melanoma is particularly common in those individuals who are most susceptible to the effects of excessive sunlight (e.g., Celts with red hair, blue eyes, and fair complexions who tan poorly and tend towards sunburn and excessive freckling, i.e., skin types I and II). It is noteworthy that the incidence of superficial spreading melanoma is more closely related to sporadic intensive exposure to sunlight, particularly isolated episodes of severe sunburn in childhood, rather than to chronic lifelong exposure as with lentigo maligna melanoma.36–39 It is more common in those who sporadically sunbathe than in long-term outdoor workers. The relationship between melanoma and sunlight is further highlighted by the increasing incidence towards the equator.40
Patients who already have one melanoma have an increased risk of developing a second independent primary tumor. The reported incidence varies from 1% to 8% of cases.41,42 It should be noted, however, that much of the literature referring to multiple melanomas does not take epidermotropic metastatic disease into account and therefore the true incidence is likely to be towards the lower figure.
There is a lack of convincing evidence that pregnancy strongly influences the outcome and prognosis of melanoma.43 Although earlier studies indicated that the mean thickness of pregnancy-associated tumors was significantly greater than that of nonpregnancy-associated melanomas, later studies have not, and the clinical outcome parallels that of nonpregnant female patients of the same age and tumor characteristics.44,45 Similarly, there is no clearly acceptable proof that the use of oral contraceptives by females increases the risk of developing melanoma.43,46
Familial melanoma accounts for between 8% and 14% of affected patients.47,48 Genes that have been implicated include CDKN2A (9p21) (the majority), and very occasionally CDK4 (12q14). The former encodes p16, which normally functions to inhibit CDK4 activity.49,50 Approximately 20–30% of patients with familial melanoma have germline mutations in CDKN2A.47,51 Familial melanoma patients are often younger than those who develop sporadic melanoma and tumors are frequently multiple. Dysplastic nevi are also present.
Melanoma shows a male preponderance (3:2) and males have a higher mortality.6,26 This probably relates at least in part to later presentation. Females generally have thinner tumors than males at first examination. The leg (particularly the calf) is the site of predilection in females, whereas the back is most often affected in males.26 Melanoma arising on the head and neck is also more common in males than in females.26
The prognosis of melanoma is to some extent site dependent. Tumors arising on the BANS sites (upper back, posterior arm, posterior neck, and posterior scalp) behave less favorably than those on the extremities.26,52
Lesions on the integument have traditionally been classified into four major clinicopathological subtypes although in the more recent literature the validity of such subdivision has been questioned, and classification into more genetically oriented groups may offer advantages.53,54 Gene profiling and the results of other high-throughput technologies will also likely aid in revising our traditional classification scheme so that clinically and therapeutically relevant subsets can be identified.55,56 An evolving molecular classification is presented in the final section of this chapter. The currently accepted subdivision is particularly based on perceived differences in prognosis and etiology. Although any variation in biological behavior relates more to tumor thickness than histogenetic subtype, there are certainly histological differences that make the distinction possible in the majority of cases. Contrariwise, in some melanomas, it is not possible to effect a precise classification and, in such instances, an unqualified diagnosis of melanoma is quite sufficient. For the moment, however, subtyping melanoma is firmly entrenched in both the literature and pathology dogma.57 Four major subtypes of cutaneous melanoma are currently recognized:19,58–63
Lentigo maligna and lentigo maligna melanoma
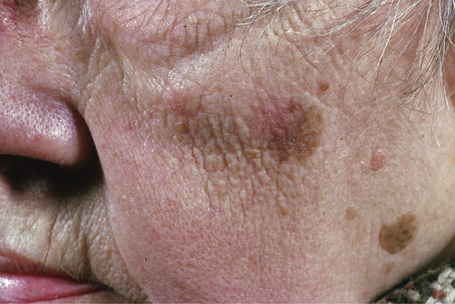
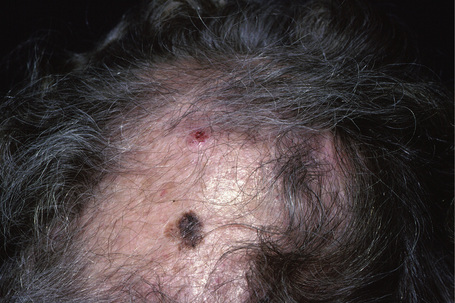
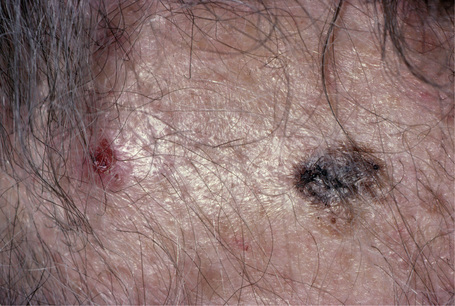
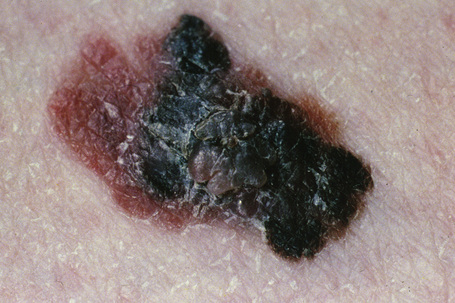
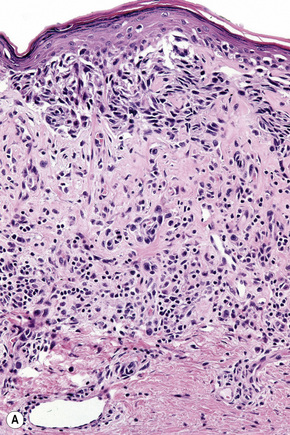
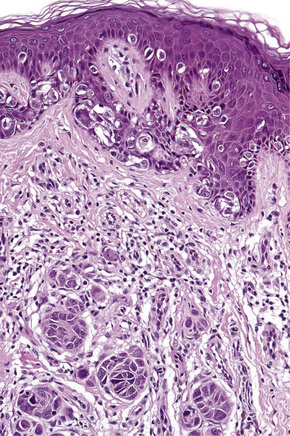
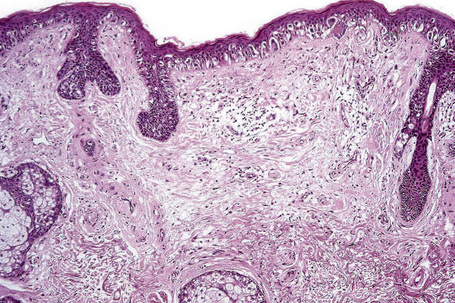
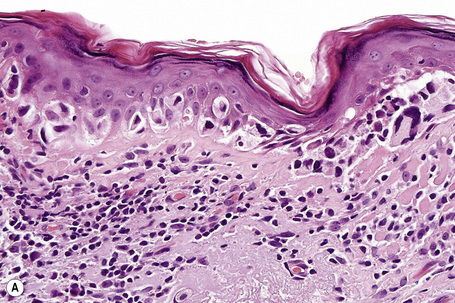
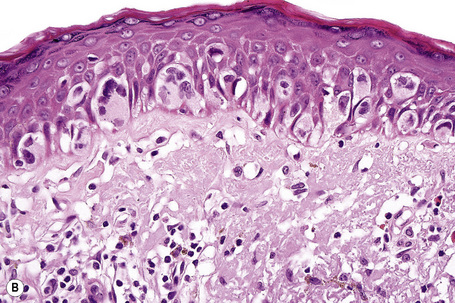
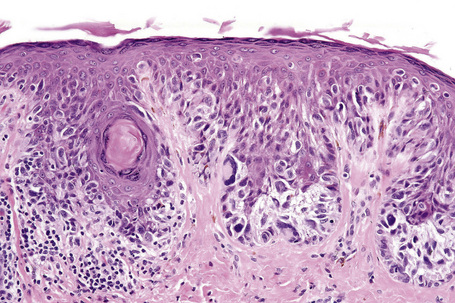
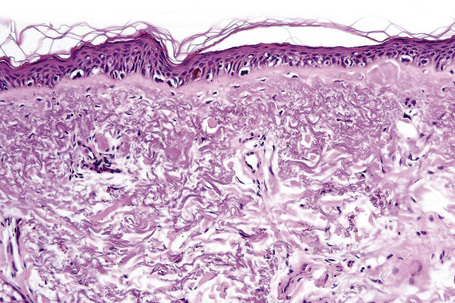
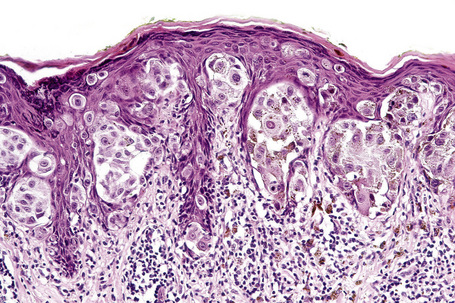
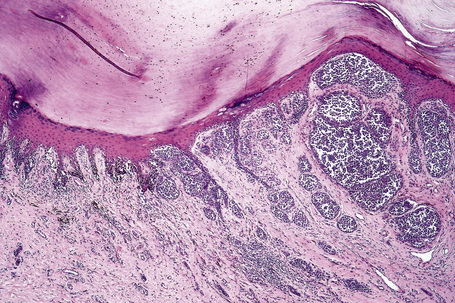
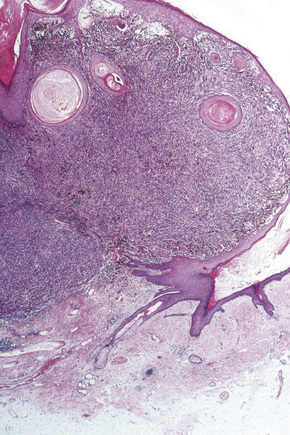
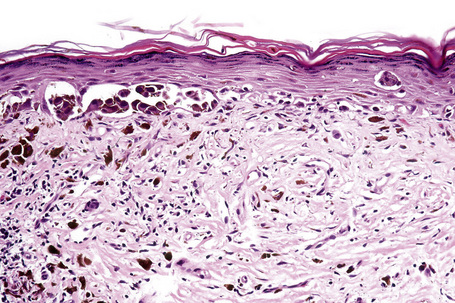
Lentigo maligna (Hutchinson’s melanotic freckle) is a relatively uncommon variant of melanoma and typically develops on chronic sun-damaged skin of the elderly.64 In the USA it is estimated to account for 4% of all cases of cutaneous melanoma. It is characterized by a positive correlation with increasing age.65 Sites of predilection are the malar region, nose, temple, and forehead; much less frequently acral sites, such as the dorsum of the hands, may be involved (Figs 26.1–26.5). The tumor presents most often in the sixth and seventh decades as a variably pigmented, gradually enlarging, irregular, flat macule. It may be brown or black and usually shows areas of hypopigmentation representing areas of regression. The in situ lesion is often present for 10–15 years before invasive tumor develops.
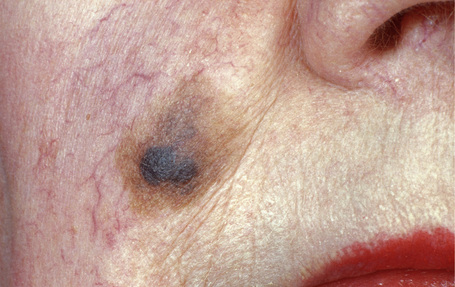
Fig. 26.2 Lentigo maligna: a dark black nodule of invasive tumor is surrounded by typical lentigo maligna.
From the collection of the late N.P. Smith MD, the Institute of Dermatology, London, UK.
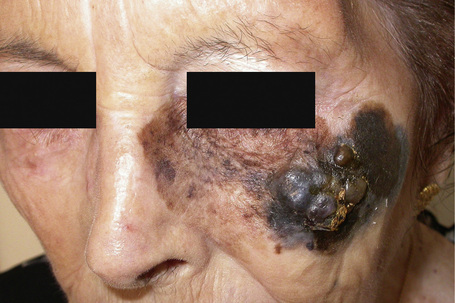
Fig. 26.3 Lentigo maligna melanoma: this is a very advanced lesion with a multinodular invasive component.
By courtesy of J.C. Pascual, MD, Alicante, Spain.
Superficial spreading melanoma
Superficial spreading melanoma is the most common variant and shows an equal sex incidence.66 The sites most frequently affected are the leg (especially in females) and the back (particularly in males). The lesion initially presents as a flat scaly macule or plaque, which after a variable period of time develops a blue or blue-black nodule of invasive melanoma (Fig. 26.6). Hypopigmented or amelanotic variants are erythematous or flesh colored. The initial plaque is irregular, often 1–2 cm in diameter, and shows much greater variation in color than a banal or dysplastic nevus. Scalloping of the border of the lesion is characteristic, and hypopigmented areas of regression are also a frequent finding. Ulceration is an important diagnostic (and prognostic) clue.
Acral lentiginous melanoma
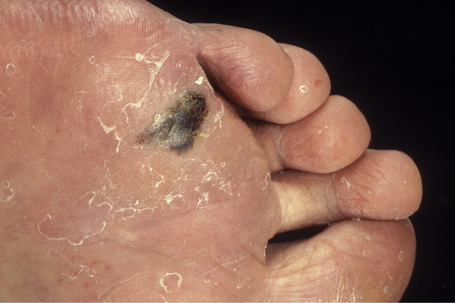
Acral lentiginous melanoma accounts for approximately 8–10% of all melanomas in Caucasians.67,68 It is, however, the predominant subtype affecting Afro-Caribbeans and Asians. The tumor is particularly found on the digits (especially beneath the nails) and on weight-bearing sites; plantar tumors are the most common, with the heel being the most frequently affected region (Figs 26.7, 26.8).69,70 Subungual variants, which most commonly affect the great toe and the thumb, are rare tumors, accounting for only 2% of all cutaneous melanomas. They show a female preponderance and present most often in the elderly.67 In addition to acral lentiginous lesions, subungual melanoma may also represent superficial spreading and nodular histological variants.71–73
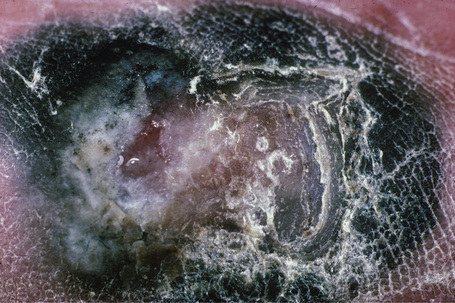
Fig. 26.8 Acral lentiginous melanoma: this ulcerated tumor had extended deeply into the underlying tissues.
From the collection of the late N.P. Smith MD, the Institute of Dermatology, London, UK.
Acral lentiginous tumors usually present as irregular, gradually enlarging, and variably pigmented macules. With progression to vertical growth phase, frequently ulcerated, blue or black nodular lesions are encountered. In Caucasians, acral lentiginous melanoma presents most often in the seventh decade, has an equal incidence in both sexes, and is generally associated with a poor prognosis since tumors are generally thick by the time of diagnosis. Mucosal melanomas are often classified within the acral lentiginous spectrum, given certain partial morphologic overlap. These two types of melanoma are distinct clinically, although molecular characterization does indicate shared features.74
Nodular melanoma
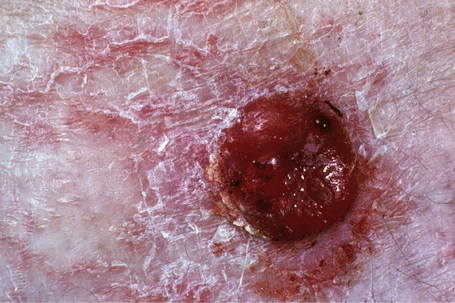
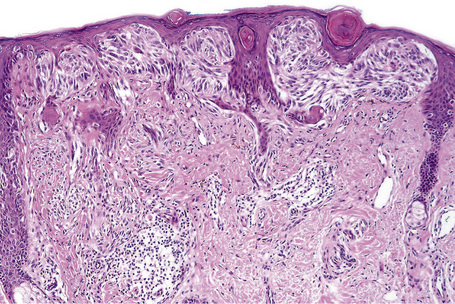
Nodular melanoma (3–4%) has no radial growth phase and therefore may be distinguished clinically from nodules of invasive tumor that have arisen in lesions associated with a pre-existent radial growth phase. It has a poor prognosis (the majority being thick tumors by the time of excision), affects more males than females (2:1), and generally arises in the fifth or sixth decade.75 The trunk and limbs are most commonly involved. The tumor, which may be nodular or polypoid (polypoid melanoma), is often ulcerated and, when lacking pigment (amelanotic melanoma), is frequently mistaken for a vascular tumor, such as pyogenic granuloma (Figs 26.9, 26.10).
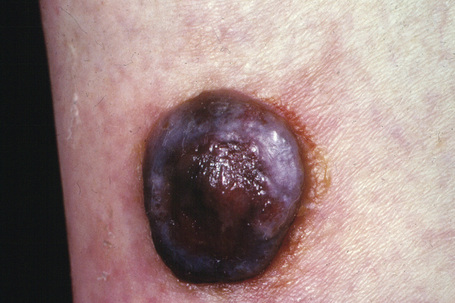
Fig. 26.9 Nodular melanoma: this heavily pigmented, dome-shaped nodule has no adjacent macular component.
From the collection of the late N.P. Smith MD, the Institute of Dermatology, London, UK.
Melanoma arising at noncutaneous (primarily mucosal) sites
Melanoma may arise at a diverse range of sites other than the skin, including the orbit, the oral cavity and nasal cavities, the external genitalia, vagina, urethra, and anus. In general, mucosal tumors are associated with aggressive behavior and a poor prognosis.76,77 This relates particularly to delayed presentation. Rare sites for primary melanoma also include the meninges, esophagus, stomach, uterus, cervix, breast, biliary system, bronchus, and adrenal gland.
Histological features
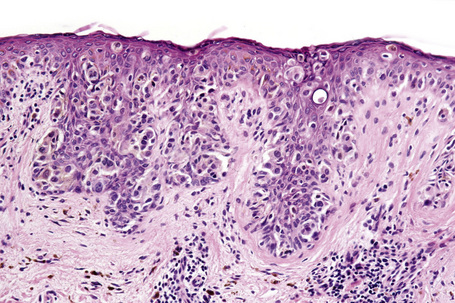
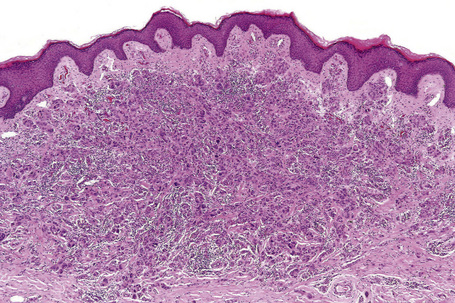
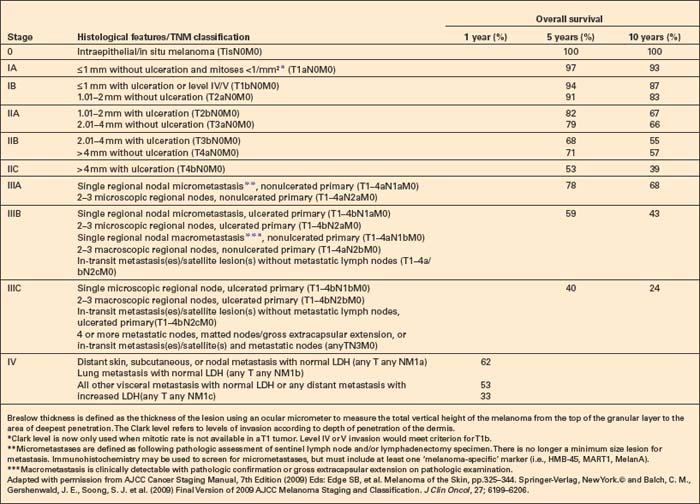
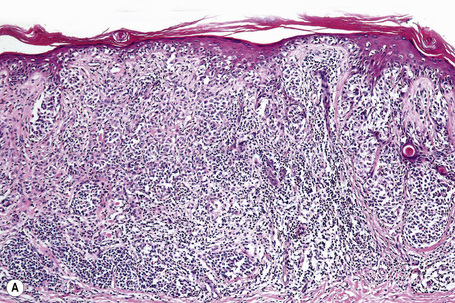
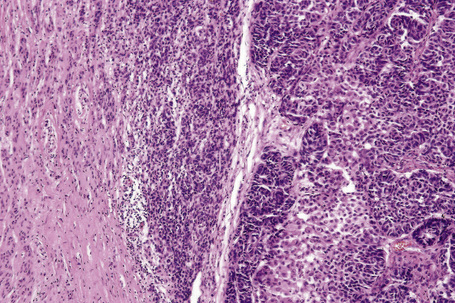
Central to our understanding of the histological classification of cutaneous melanoma is the concept of radial (horizontal) growth phase and vertical (invasive) growth phase.23,59,78,79 By current definition, the radial growth phase may include (in addition to a wholly in situ, intraepidermal component) evidence of microinvasion into the papillary dermis (microinvasive radial growth phase), often accompanied by features of regression (Figs 26.11, 26.12). The microinvasive stage of melanoma is believed to lack significant metastatic potential and as a consequence is associated with an excellent prognosis.80,81 Indeed, in a large series of patients discussed by Clark and coworkers, no tumors in the radial growth phase were associated with metastatic spread.60
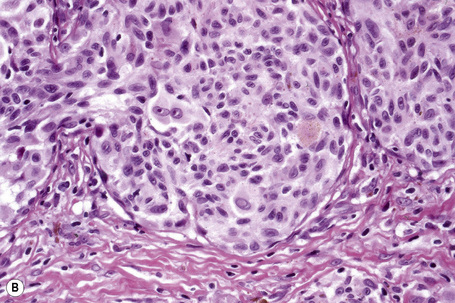
Histologically, the microinvasive radial growth phase tumor is characterized by single cells or small aggregates of melanoma cells, histologically similar to their intraepidermal counterparts and invariably forming tumor nests smaller than those present within the overlying epidermis.23 A lymphohistiocytic infiltrate is usually present. Mitotic figures are invariably absent. The last feature is of particular importance; multiple levels should therefore be carefully examined before making a diagnosis of microinvasive radial growth phase melanoma (Fig. 26.13).
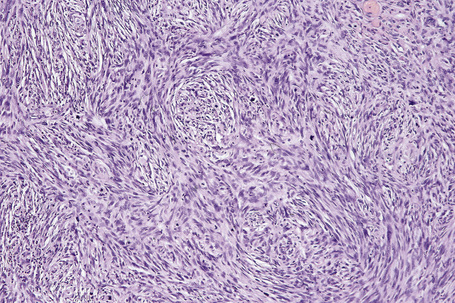
Vertical growth phase melanoma is composed of cohesive nests, nodules or plaques larger than those present within the epidermis and consisting of tumor cells that are cytologically different from those in the radial growth phase (Fig. 26.14).23,79 Mitotic figures are common.23 Features of regression may be seen but are usually absent at the base of the tumor. The tumor cells in the vertical growth phase are pleomorphic and apoptosis is often present. Vertical growth phase implies an alteration in biological potential with a capacity for lymphovascular invasion and metastatic spread.23,79,80
Lentigo maligna and lentigo maligna melanoma
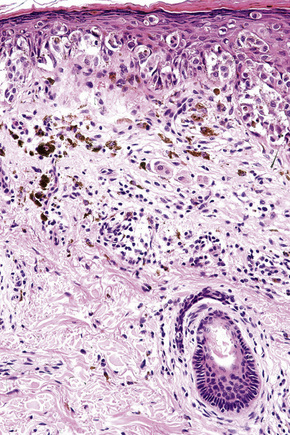
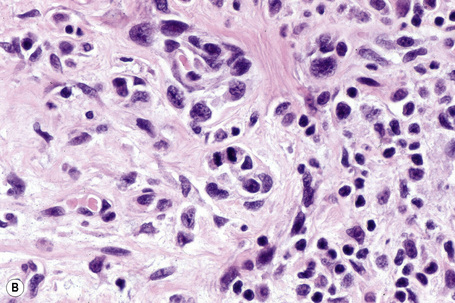
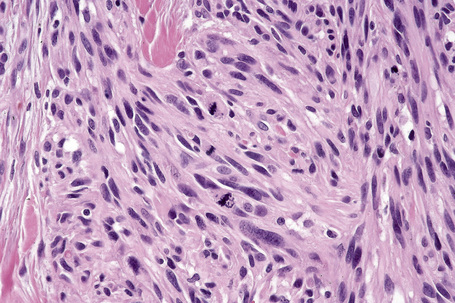
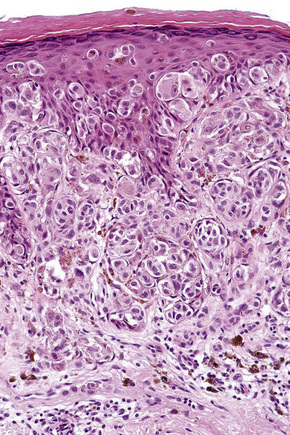
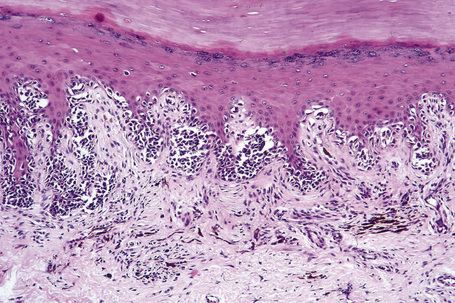
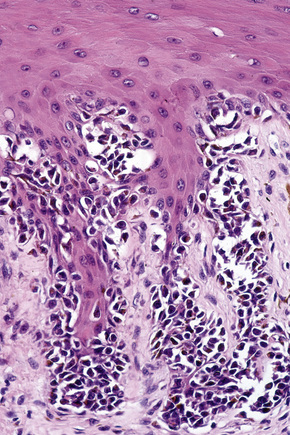
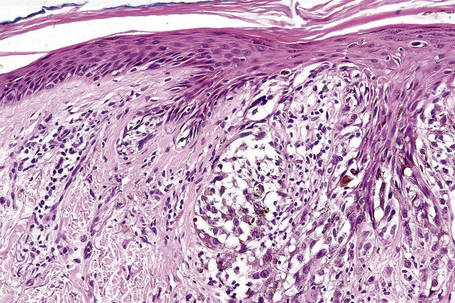
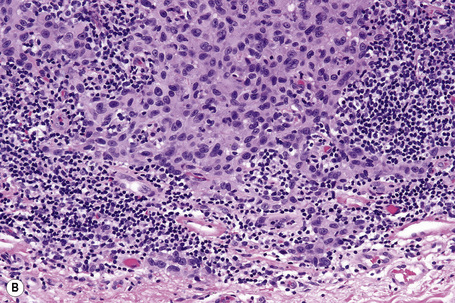
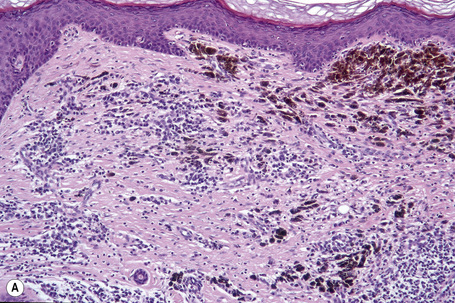
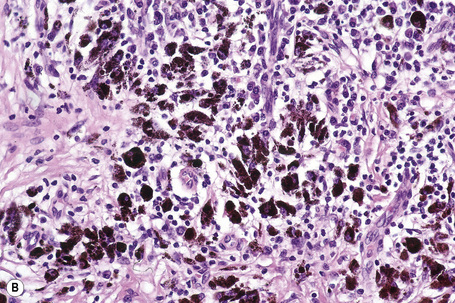
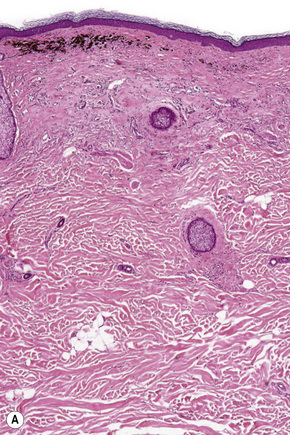
Lentigo maligna is characterized by proliferation of atypical melanocytes predominantly located along the dermoepidermal junction (Figs 26.15, 26.16).82 The tumor cells often show a conspicuous cytoplasmic fixation retraction artifact and contain pleomorphic irregular hyperchromatic, sometimes angular, nuclei. The cells are frequently orientated perpendicular to the surface, and involvement of hair follicles and sweat duct epithelium is a characteristic finding (Fig. 26.17). This may sometimes result in difficulties in assessing tumor thickness, particularly if obliquely cut sections are examined, when the relationship of the tumor cells to the adnexal epithelium may not be clear. In more advanced lesions, junctional nests are apparent and multinucleate tumor giant cells are commonly seen (Figs 26.18–26.20). Pigmentation is variable, but is often abundant, sometimes involving the full thickness of the epidermis, including the stratum corneum. The occasional presence of marked intraepidermal (pagetoid) spread may result in histological overlap with superficial spreading melanoma. Assessing excision margins for residual atypical melanocytes in this variant is often fraught with difficulty. To avoid unnecessary surgery it is important not to confuse actinically damaged nuclei from residual lentigo maligna (Fig. 26.21).
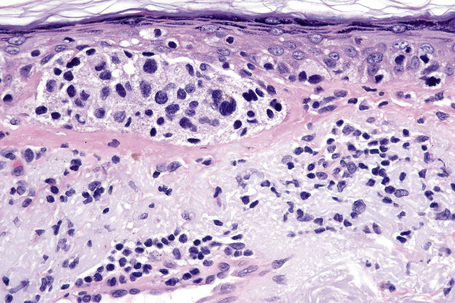
Fig. 26.19 Lentigo maligna: the tumor cells are pleomorphic and show very marked nuclear hyperchromatism.
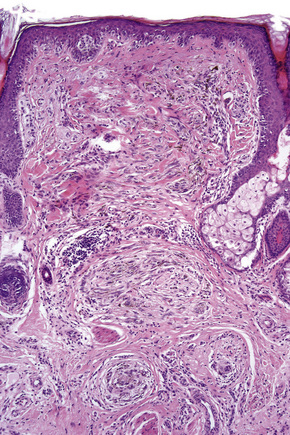
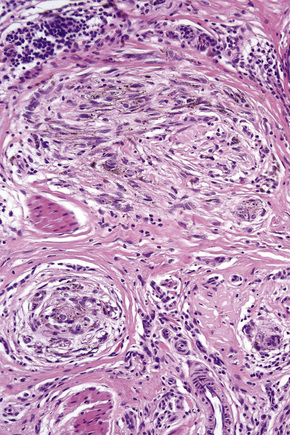
Lentigo maligna arises at sites showing actinic damage; the epidermis is therefore typically atrophic and the dermis shows solar elastosis. Usually the papillary dermis contains melanophages and scattered chronic inflammatory cells. The finding of the latter, particularly when present in large numbers, is often associated with invasion and should therefore prompt careful examination of multiple levels of the specimen. Invasive tumor (lentigo maligna melanoma) can be multifocal and is usually of the spindled cell type (Figs 26.22–26.24). Desmoplasia, often with neurotropism, is present in a significant percentage of cases. Very occasionally, a storiform growth pattern is evident and if the tumor is amelanotic there may be confusion with dermatofibrosarcoma protuberans, particularly in small biopsies (Fig. 26.25). More often, the associated melanoma is desmoplastic with subtle spindle cells and can be mistaken for fibrosis or scar rather than invasive tumor.
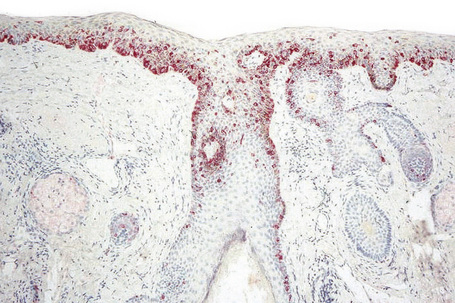
Exceptionally, lentigo maligna and lentigo maligna melanoma present as amelanotic lesions (amelanotic lentigo maligna and amelanotic lentigo maligna melanoma), both clinically and histologically.83–85 Clinically, these appear as erythematous scaly lesions resembling actinic keratoses, squamous cell carcinoma in situ or eczema.84 Immunocytochemistry may therefore be necessary to arrive at the correct diagnosis (Fig. 26.26).86
Superficial spreading melanoma (pagetoid melanoma)
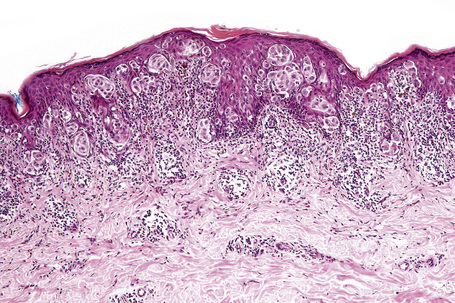
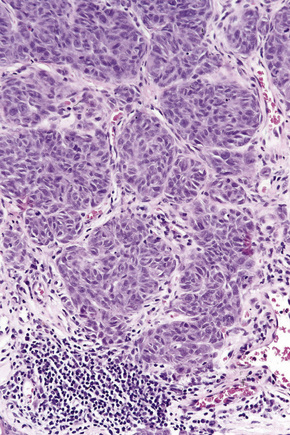
The radial growth phase of superficial spreading melanoma is now encountered more commonly, largely as a consequence of public awareness of melanoma with the consequent removal of an increasing percentage of thin early lesions. It is typified by an asymmetrical proliferation of atypical nondendritic melanocytes scattered singly and in clusters throughout all levels of the epithelium (buckshot scatter), giving an appearance reminiscent of Paget’s disease (Figs 26.27, 26.28). The melanoma cells involve all layers of the epithelium while the cells of Paget’s disease tend to be superficial to the basal cell layer. The individual cells are epithelioid with abundant cytoplasm, often showing fine or dusty melanin pigmentation, and contain pleomorphic vesicular nuclei with prominent eosinophilic nucleoli; scattered mitotic figures including atypical forms may be evident. Characteristic of this variant is tumor growth in continuity from one rete ridge to another (a pattern not usually seen in acquired junctional or compound nevi). In contrast to lentigo maligna, there is often little visible evidence of actinic damage. The epidermis often shows acanthosis with partial or complete effacement of the ridge pattern. Invasive tumor is mostly of the epithelioid type (Figs 26.29–26.32). Desmoplasia and/or neurotropism are uncommon.
Acral lentiginous melanoma
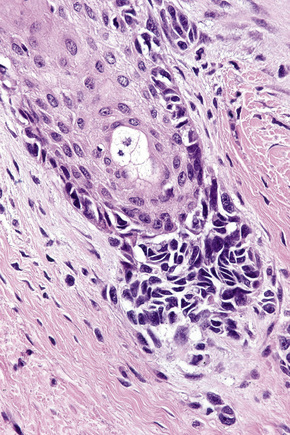
In the early stages of the radial growth phase of acral lentiginous melanoma the changes may be quite subtle, consisting of irregular epidermal hyperplasia and scattered, basally located, atypical melanocytes.87–90 The established lesion shows acanthosis with marked elongation of the epidermal ridges and obvious melanocytic atypia (Figs26.33, 26.34). The lower reaches of the epidermis are infiltrated by large numbers of atypical melanocytes characterized by nuclear pleomorphism and hyperchromatism, and showing a cytoplasmic fixation retraction artifact. Nucleoli are conspicuous and mitotic figures may be identified. Although spindled forms are most often encountered, epithelioid and giant cells are sometimes evident. Scattered foci of junctional nests may also be detected, usually at the tips of the epidermal ridges (Fig. 26.35). A heavy bandlike chronic inflammatory cell infiltrate is frequently present. The invasive tumor is often a spindled cell in type and commonly elicits a desmoplastic reaction (Fig. 26.36). Deep extension along the sweat gland epithelium is common and neurotropism may be evident in a subset of cases. Occasionally, acral tumors may show a superficial spreading in situ component or represent de novo nodular melanoma with absent radial growth phase.
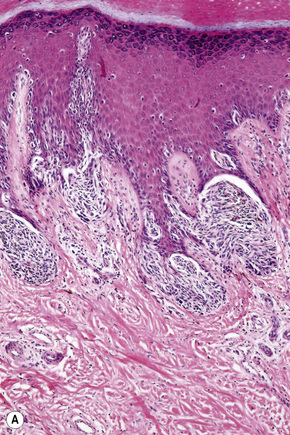
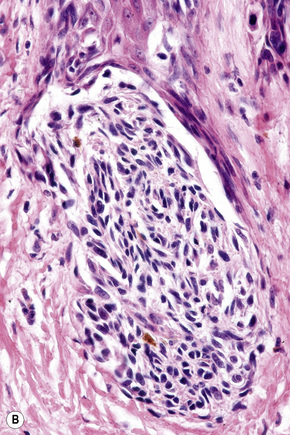
Fig. 26.35 (A, B) Acral lentiginous melanoma: large junctional nests are present at the tips of the rete.
Nodular melanoma
By definition, nodular melanoma has no evident preceding radial growth phase, its growth pattern appearing vertical from inception. Such a tumor, therefore, does not show an intraepidermal melanocytic proliferation beyond three epidermal ridges on either side of the tumor mass (Figs 26.37, 26.38). This histologic pattern is usually, but not always, seen in the same anatomic distribution as superficial spreading melanoma.
Cell types
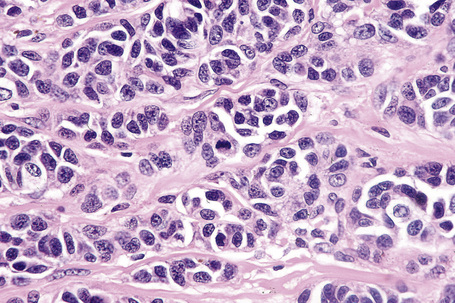
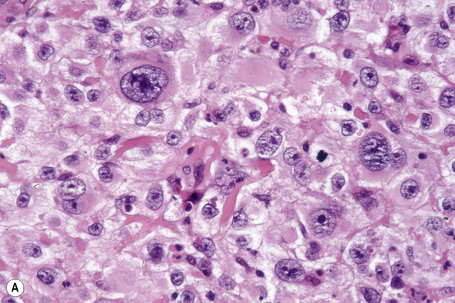
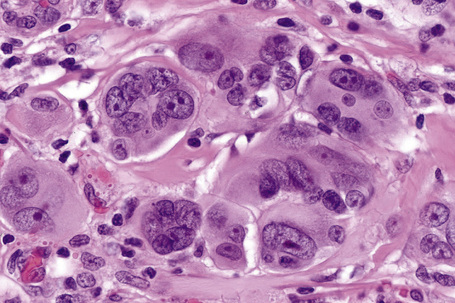
Epithelioid cells
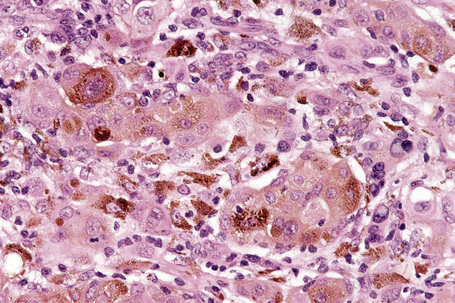
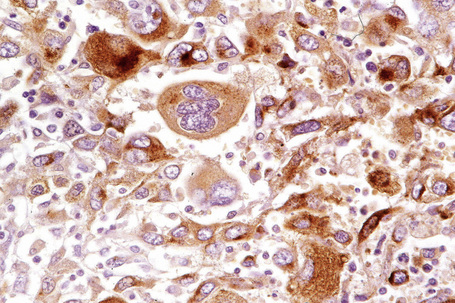
Epithelioid melanoma cells are large and rounded with abundant, often eosinophilic, cytoplasm and contain prominent pleomorphic vesicular nuclei with conspicuous eosinophilic nucleoli. Mitotic figures may be either scant or numerous and sometimes abnormal. Pigmentation is variable and can be abundant or minimal. When minimal, Masson-Fontana silver staining is useful to reveal small quantities of pigment not detectable with conventional hematoxylin and eosin stained sections. Alternatively, immunohistochemistry may be necessary to establish the diagnosis (Fig. 26.39).
Spindled cells
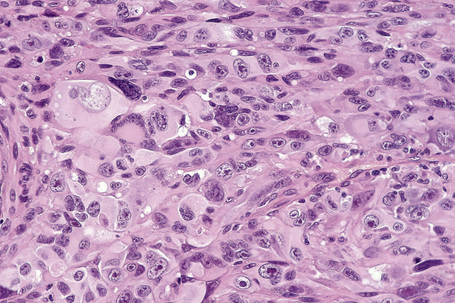
Occasional melanomas are exceedingly pleomorphic, containing tumor giant cells with enlarged nucleoli and conspicuous, frequently abnormal, mitotic figures; these variants must sometimes be distinguished from metastatic tumors, including pleomorphic secondary carcinoma, sarcomas, and even anaplastic CD30+ lymphoma (so-called giant cell melanoma) (Figs 26.40–26.42).
Prognostic indicators
Clinical prognostic indicators include age, sex, and site of the primary tumor.91–94 Older patients fare worse than younger ones, and males have a poorer outlook than females.7,93–98 The latter is independent of tumor thickness and site.93 Particularly high-risk sites include the back, upper arm, neck, and scalp (BANS).99 The acral sites are also thought to be associated with a poorer prognosis.100
When reporting melanoma, it is generally accepted as essential worldwide to record and comment on the following variables:93,101–104
Growth phase is also noted although it should be emphasized that great care must be taken before allocating a melanoma to the microinvasive category of radial (horizontal) growth phase. Dermal mitoses automatically place a tumor in the vertical growth phase (see Fig. 26.14). These elements have become critical for proper staging and prognostic assignment of patients, but unfortunately even in countries with a high incidence and acute awareness of cutaneous melanoma, many of the above elements are absent from routine reports.105
Breslow tumor thickness is the most important single prognostic indicator.106 It is measured from the most superficial aspect of the granular cell layer to the deepest point of invasion of the tumor. In ulcerated tumors, the measurement should be from the base of the ulcer. The significance of periadnexal extension is less clear. If the latter is greater than the conventional Breslow thickness, the information should also be documented in addition to the traditional Breslow measurement (Fig. 26.43). Polypoid tumors should be treated no differently, and should be measured through the thickest region.107 Tumor volume, therefore, is a most important factor influencing outcome in vertical growth phase melanoma. Careful evaluation of the greatest tumor thickness provides very useful prognostic guidance.108–111 In the latest (7th) edition of the American Joint Committee on Cancer (AJCC) staging system (2009, effective in 2010), the thickness thresholds have been maintained as in the 7th Edition at 1.00, 2.00 and 4.00 mm (Table 26.1).112–115 While certainly not perfect, clinical and particularly micropathological staging in melanoma is a model for other cancers.
The level of tumor invasion when classified according to Clark levels is as follows:
The dermal interface may be identified by the site of the superficial capillary plexus. It also corresponds to the zone of transformation of the horizontally orientated reticular dermis elastic fibers to the vertically aligned ones of the papillary dermis. The distinction between Clark level II and III and perhaps more so between III and IV is somewhat difficult to apply in practice and is observer dependent.116 Clark level was believed to provide independent prognostic information for thin tumors (1.00 mm or less in thickness) but not for thicker melanomas.117,118 The latest data indicate that if mitotic rate is assessable in thin melanomas, then Clark level no longer provides additional prognostic information. The 2009 (7th) AJCC staging system no longer recommends the use of the Clark level, even for thin melanomas, if a mitotic rate in the dermal component is available.113,114
The presence or absence of ulceration and the width of the ulcer are independent prognostic indicators and should be recorded.92 Ulceration is defined as ‘the absence of an intact epidermis overlying a major portion of the primary melanoma based on microscopic examination of the histologic sections’.113 Trauma and artifactual loss of the epidermis must be excluded. Ulceration is associated with a very significant increased risk of metastasis and was first included as a second determinant in the T classification in the AJCC 6th Edition.113,117–119
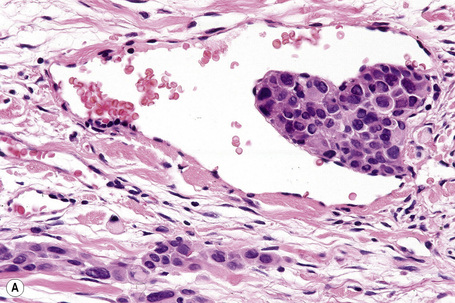
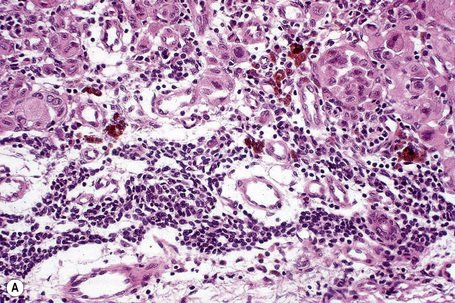
Tumor-infiltrating lymphocytes are an important independent prognostic variable and should be recorded as brisk, nonbrisk or absent (Figs 26.44–26.46).79,120–122 The brisk category implies lymphocytes present throughout the whole vertical growth phase or extending across its entire base. Nonbrisk tumor infiltrating lymphocytes implies focal infiltration only. Absent includes two categories: either no lymphocytes at all or lymphocytes are present but do not infiltrate the melanoma. Brisk lymphocytic responses tend to be a feature of thin melanomas whereas absence of a lymphocytic response is generally seen in thick melanomas.110,121 In a study of 285 vertical growth phase tumors, the 10-year survival rates for brisk, nonbrisk, and absent tumor-infiltrating lymphocytes were 55%, 45%, and 27%, respectively.121 Not surprisingly, the absence of tumor-infiltrating lymphocytes also correlated with metastasis to sentinel lymph nodes as a surrogate marker of melanoma risk in a cohort of 887 patients with full multivariate analysis.122 Assessing tumor-infiltrating lymphocytes in lymph node metastases may also have predictive value.123
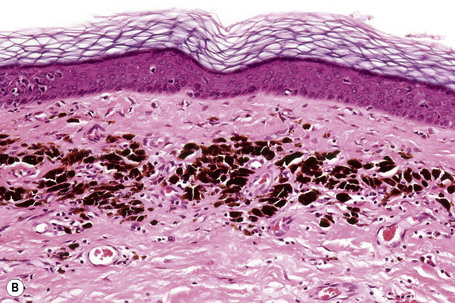
In thin melanoma it is important to recognize the features of regression, which may be particularly evident in the dermal component (Figs 26.47, 26.48).79 These include absence or reduced numbers of malignant melanocytes, degenerate (apoptotic) forms, and a chronic inflammatory cell infiltrate (Fig. 26.49).124–126 Melanophages, horizontal scarring, isolated tumor islands, and telangiectatic vessels are also commonly present in the later stage. Clinically, regression presents as macular gray, white or pink areas.
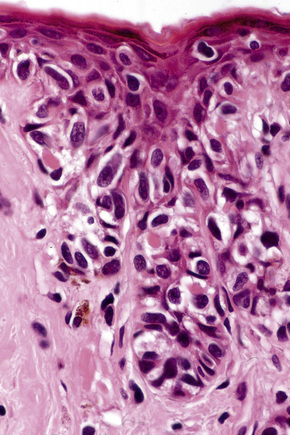
Fig. 26.49 Regression: in this example, there is conspicuous apoptosis. Note the eosinophilic bodies.
Although the importance of regression as a determinant of biological behavior has been the subject of considerable argument in the literature, a number of authors believe that, in thin tumors, it correlates with an impaired prognosis, though this contribution may be minor relative to other factors in many cases.126–134 Complete regression of an undiagnosed primary melanoma may be the explanation for patients presenting with metastatic tumor of indeterminate derivation (Fig. 26.50). Some authors recommend sentinel node biopsy for thin melanomas if there is evidence of extensive (> 50%) regression.93
Mitotic rate is determined by the number of mitotic figures/1 mm2 of tumor in the most mitotically active area. It has become clear that tumors displaying a high mitotic rate are associated with a poorer prognosis and that mitoses are a more robust predictor of outcome than ulceration and thus mitotic rate has been incorporated into the 7th AJCC staging system.79,114,135–140 Despite earlier contrary evidence and the complex interrelations of tumor thickness, ulceration, and mitotic rate, mitotic rate appears to carry prognostic information independent of both of the other factors.93,141,142
The presence of a microscopic satellite is defined by a distinct tumor nodule measuring 0.05 mm or more in diameter separate from the main tumor mass in the section containing the thickest region of the melanoma. It is found in thicker tumors and is associated with an increased risk of local recurrence, regional lymph node metastases, and diminished survival.143–145 The presence of microsatellites is recorded as N2c (without positive lymph nodes) and N3 when seen in combination with positive lymph nodes in the 2009 AJCC melanoma staging system.113
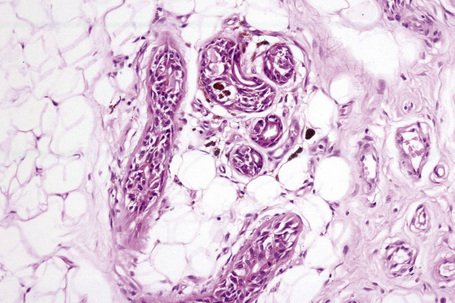
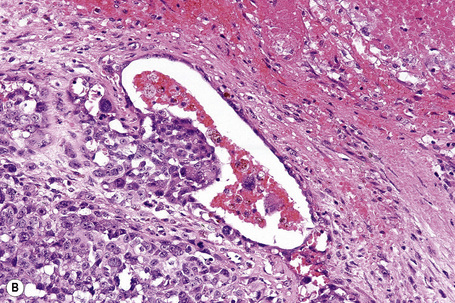
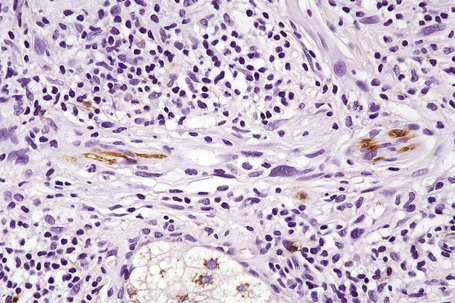
The presence of lymphatic invasion correlates with the development of in-transit metastases (Fig. 26.51).146 Lymphovascular invasion has been shown to represent a predictor of diminished survival in melanoma in a number of studies.122,147–50 The use of immunohistochemistry for D2-40 significantly increases the sensitivity for detection of lymphatic invasion and also correlates with lymph node metastasis and survival.151 More study is needed to validate the application and interpretation of the technique. Contrariwise, absence of vascular involvement in thick melanomas correlates with increased survival in a number of studies.152,153 Perineural and intraneural infiltration are most often encountered in desmoplastic variants, thereby accounting, in part, for the increased risk of local recurrence (Fig. 26.52). In this context, CD57 immunohistochemistry can be helpful in identifying residual nerve (Fig. 26.53).
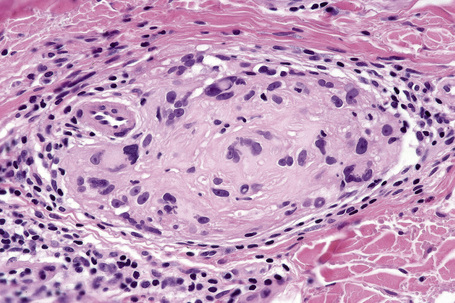
Fig. 26.52 Melanoma: intraneural invasion. Note the pleomorphic tumor nuclei within this nerve trunk.
Angiogenesis, which is defined as the increasing development of new blood vessels at the base of the melanoma, parallels increases in tumor thickness.154–157 Increasing angiogenesis therefore correlates with thick tumors, ulceration, relapse, and tumor-associated death.157,158 Perhaps related to the likelihood of lymphovascular invasion, increased intratumoral lymphangiogenesis as measured by D2-40 or VEGF-C also correlates with metastasis to sentinel lymph nodes and reduced disease-specific survival.159
Sentinel node biopsy also adds extremely valuable prognostic information.160–164 It has been shown to represent the most important factor in determining likelihood of tumor recurrence and patient survival in stage I and stage II disease.161 Whether the procedure has therapeutic value is uncertain but it can spare the patient unnecessary regional lymph node dissection.163 The latest AJCC staging system recommends incorporation of sentinel lymph node data in cases where it would inform management, as this procedure significantly increases the accuracy and discriminatory power of the stage groups.114 Lymph nodes are bivalved or serially sectioned along their long axis and in addition to the examination of hematoxylin and eosin stained sections, immunohistochemistry – such as S-100 protein, HMB-45 and/or MART-1 (melanoma antigen recognized by T cells 1) – should invariably be performed in those cases where the initial sections are negative. Detection of single or tiny groups of cells using an antibody against a ‘melanoma-specific’ antigen such as HMB-45 or MART1 is now incorporated into the AJCC staging system. More recently, molecular techniques including reverse transcriptase polymerase chain reaction for tyrosinase messenger RNA have been proposed.165,166 A major disadvantage of such procedures is the likelihood of false-positive results due to the common presence of banal capsular nevi, though the use of markers more specific to melanoma over nevus cells could potentially alleviate this problem.167
Sentinel node biopsy is currently recommended for all tumors measuring 1.00 mm or more in thickness. Other possible indications include ulcerated tumors, tumors with 50% or more regression, tumors having achieved the vertical growth phase, and those lesions which have been biopsied and involve the deep margin.93,163,168 The procedure should also be considered for tumors that are less than 1.00 mm thick but are Clark level IV or with conspicuous mitotic activity.169
Immunohistochemistry of melanoma
Immunohistochemistry is a valuable adjunct to histology in the diagnosis of melanoma, particularly in amelanotic, epithelioid, and spindled cell variants and their distinction from undifferentiated carcinomas and mesenchymal tumors.1,2 Owing to problems of specificity and sensitivity, it is prudent to use two or even three ‘melanoma markers’ in such problematical cases. Using these markers as part of a panel looking at multiple lines of differentiation is also of practical use, such as inclusion of keratins to exclude epithelial tumors. In morphologically challenging cases, a panel of stains that supports the ultimate diagnosis by their pattern of reactivity or nonreactivity is very helpful. The role of immunohistochemistry is to provide supportive data. It should rarely if ever be used as the sole criterion by which a diagnosis of melanoma is achieved.
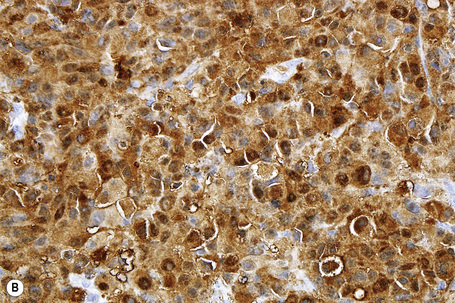
S-100 protein remains the yardstick in the immunohistochemical diagnosis of melanoma.3–5 Although there are now substantial numbers of new markers available, none as yet, in isolation, measures up to S-100 protein. However, S-100 protein lacks specificity and there are very exceptional S-100 protein-negative melanomas. In cases where the diagnosis remains in doubt, use of a battery of the newer immunohistochemical markers may be of great value.1
S-100 protein is a calcium binding F-band protein, isolated from brain. It is variably positive in 94–100% of primary and metastatic melanomas.1,2 In addition to melanocytes, Schwann cells, myoepithelial cells, adipocytes, chondrocytes, Langerhans cells, and tumors derived thereof, express S-100 protein. Staining of Langerhans cells can sometimes be a problem, particularly when assessing the extent of intraepidermal melanocyte spread. In such instances, the addition of HMB-45 or MART-1 may be helpful. S-100 protein may also be expressed in a number of breast carcinomas and undifferentiated carcinomas.4,5 The most commonly employed antibody against S-100 is a purified rabbit polyclonal antibody against S-100 protein purified from bovine brain. More than 20 members of this family exist and monoclonal antibodies are available for many of them. While not well established in large series, there may be some selectivity of the isoforms between melanoma and other traditionally S-100 protein reactive neoplasms in the differential diagnosis.6
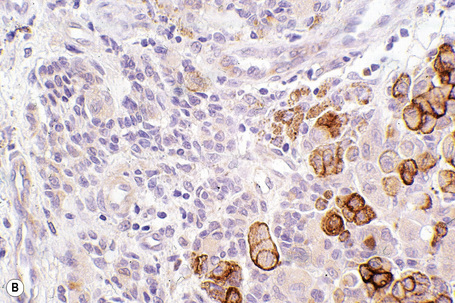
HMB-45 reacts with the cytoplasmic premelanosome glycoprotein gp100 and is less sensitive than S-100 protein.6 It is expressed by 80–86% of metastatic melanomas and between 90% and 100% of primary tumors.1,3,6 However, expression is often more focal than with S-100. Sensitivity diminishes in spindled cell variants and it is usually negative in desmoplastic melanoma (see below).7,8 The junctional and superficial dermal components of banal nevi also react with HMB-45 but the deeper dermal nevus cells are generally negative. This differential staining pattern may be of value in confirmation of pre-existent banal nevus cells associated with a melanoma and in particular their distinction from small cell and nevoid melanoma, which are typically positive in the deepest nests of cells (Fig. 26.54). It is very important to remember that this pattern is lost in cases where melanocytes are pigmented as the latter are positive for HMB-45. Dysplastic nevi are similarly labeled. Blue nevi and deep penetrating nevi are also HMB-45 positive.1 Spitz nevi are often HMB-45 positive in the superficial aspect of the lesion and this is usually, but by no means always, lost with depth. This finding may be of value in its distinction from spitzoid melanoma in which staining is typically present throughout the tumor. Although it is more specific than S-100 protein, HMB-45 also reacts with the group of perivascular epithelioid cell tumors (PEComas) including angiomyolipoma, lymphangiomyomatosis, and clear cell sugar tumor of the lung.9,10
MART-1 (Melan-A) is a melanosomal differentiation antigen recognized by autologous cytotoxic T cells.11–17 Some antibodies raised to this protein (e.g., A103) label a variety of lesions including adrenocortical, Leydig cell, Sertoli cell, and granulosa cell tumors, and tumors in the PEComa group of lesions including angiomyolipoma, lymphangiomyomatosis, and clear cell sugar tumors of the lung in addition to melanoma.15,16 It has a similar sensitivity to S-100 protein in epithelioid melanomas but is less sensitive in spindled cell tumors and is not usually expressed in the desmoplastic variant. A study has shown evidence that diminished MART-1 expression correlates with increasing tumor thickness, reduced disease-free interval, and increased patient mortality.15 MART-1 expression has also been demonstrated in compound, dermal nevi and Spitz nevi with the exception of neurotized variants.12–14
Antityrosinase antibody (e.g., T311) appears to be less sensitive than either S-100 protein, HMB-45 or Melan-A (A103).18–23 It does not appear to label desmoplastic melanoma.
Microphthalmia transcription factor (MITF) is a transcriptional regulator important for tyrosinase expression.24 It is strongly positive in nevi and epithelioid melanoma.25–27 Sensitivity is reduced in spindled cell and desmoplastic variants.28 Spitz nevi and neurotized banal nevi show diminished expression. The specificity of MITF is low and this limits its use in the differential diagnosis of mimickers of melanocytic lesions.
Recently, a new monoclonal antibody, SM5-1, was created by a subtractive immunization protocol using human melanoma samples and appears to bind a variant of fibronectin.29,30 Initial reports indicated that its sensitivity is similar to S-100 protein with improved specificity for other traditionally S-100 protein reactive, but the antibody also reacts with hepatocellular carcinoma and breast cancer cells.23,31 SOX10 has recently between described as a novel marker of melanocytic and Schwann cells. While it lacks specificity and stains other cells it may be useful in desmoplastic melanoma.32 Additional study and confirmation of both these new markers is necessary. The bar is high for inclusion of new markers as standards for melanoma in clinical practice. All antibodies have a natural history of decreasing specificity with study of additional tumor types and new antibodies are not as far down this curve as older antibodies, creating potential for misinterpretation if the new reagents are used alone.
Melanoma cells can express epithelial markers including keratins, EMA, and CEA.2,33–35 Keratin may be identified in as many as 10% of melanomas, both on frozen and on paraffin-embedded sections.2 CEA is commonly encountered if polyclonal antibodies are utilized.34,35 Metastatic disease more often shows such aberrant staining patterns than primary tumors. Diagnostic difficulties are unlikely to be encountered provided S-100 protein and/or other melanoma markers have been included in the antibody panel. Smooth muscle actin (SMA) and desmin are very rarely expressed in melanoma with the exception of the desmoplastic variant where, as in most spindle cell tumors, SMA can be detected to varying degrees.36 One study with dual labeling for S-100 protein and SMA in desmoplastic melanoma indicates that the SMA reactivity may be in accompanying stromal myofibroblasts.37
Melanoma cells may express histiocytic markers such as CD68 (KP1) in 80% or more of tumors.38,39 Mac 387 and α1-antitrypsin may also be positive. This is of particular significance since the distinction between tumor cells and histiocytes, particularly in sentinel lymph node specimens, can sometimes be problematical. In addition, distinguishing between balloon cell melanocytic lesions and xanthomatous infiltrates may require immunohistochemical confirmation, particularly if no residual recognizable melanocytic component is visible. In such circumstances, positive melanocytic markers are obviously of major diagnostic importance.
Recently, there has been an ever-expanding range of reputed immunohistochemical prognostic markers.40 Most of these lack appropriately powered full multivariate analysis linking the marker with specific outcome such as melanoma-specific mortality and supplemented by hazard ratios or do not fully describe the methods utilized as recommended by the NCI-EORTC reporting recommendations for tumor marker prognostic studies.41,42 The more useful of these are very briefly discussed below. Few of these are validated to the level necessary for routine application to clinical samples; virtually all are reported in retrospective cohorts. Use of multiple markers and application of rigorous methods of quantification have efficacy, distinguishing different prognostic groups.43 The markers discussed below are primarily used to help support a diagnosis of melanoma or nevus in the relatively small subset of cases where this determination is challenging on purely histologic grounds.
Ki-67 (MIB-1) is a particularly valuable adjunct in the distinction between benign melanocytic nevi (including Spitz nevi) and melanoma.44,45 In nevi, less than 5% of nuclei are positive (and these are usually located in the most superficial aspect of the dermal component) whereas in melanoma 25% or more of cells are labeled (Figs 26.55, 26.56). Its role in predicting biological behavior is controversial; thus, although in earlier studies increased expression in thick tumors was thought to correlate with poor survival, more recently it has been claimed that increased expression in thin tumors (< 1.5 mm) is of greater significance.46–49 A current study indicates that increased MIB-1 reactivity is a poor prognostic factor in terms of disease-specific survival independent of tumor thickness.50 Obviously, there is a proportional, though not exact, relationship between degree of nuclear MIB-1 reactivity and mitotic rate, the latter now being incorporated into the most recent 7th Edition of the AJCC staging system. How to correlate Ki-67 reactivity with mitotic rates in a fashion relevant to the current staging system is evolving. Increased Ki-67 expression also correlates with overexpression of p53 protein and loss of p16 (see below).48,50
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree