Learning Outcomes
After completing this chapter, you will be able to
 List eleven different types of medication errors.
List eleven different types of medication errors.
 Identify causes or factors that contribute to medication errors.
Identify causes or factors that contribute to medication errors.
 List five “high alert” medications.
List five “high alert” medications.
 Describe methods of preventing medication errors from occurring.
Describe methods of preventing medication errors from occurring.
 List examples of common medication errors.
List examples of common medication errors.
 Describe the possible consequences of actual medication errors.
Describe the possible consequences of actual medication errors.
 Explain the steps to be taken when an error has been identified.
Explain the steps to be taken when an error has been identified.
 Explain the role of quality assurance monitoring of medication errors.
Explain the role of quality assurance monitoring of medication errors.
Key Terms
| compliance error | An error occurring when patients do not follow their dosing regimen. |
| deteriorated drug error | Use of an expired medication or one whose properties have been compromised. |
| failure mode and effects analysis (FMEA) | A process that evaluates where errors might occur and estimates their potential impact. |
| high alert medications | Medications that have a high risk of causing patient harm when used in error. |
| improper dose error | A dose that is greater than or less than that ordered by the prescriber. |
| medication error | Any error occurring in the medication use process. |
Compounding/Drug Preparation Errors
Work Environment and Personnel Issues
Deficiencies in Medication Use Systems
Prevention of Medication Errors
Failure Mode and Effects Analysis
Systems Designed to Prevent Medication Errors
Computerization and Automation What to Do When an Error Occurs Monitoring the Impact of Change |
|
Pharmacists are responsible for the safe and appropriate use of medications in all pharmacy practice settings. As part of the multidisciplinary health care team, the pharmacist’s role is to establish patient-specific drug therapy regimens designed to achieve predefined therapeutic outcomes without subjecting the patient to undue harm.
As pharmacists become more involved in patient-specific care, technicians are permitted to perform tasks that were previously restricted to pharmacists. As their responsibilities expand, the role of technicians in ensuring medication safety also increases. As a result, they need to be aware of potential causes of medication errors and the significance of their role in preventing those errors.
Numerous terms are used to describe drug-related incidents. The term medication misadventure is used to describe adverse drug reactions (unintended responses to drugs used at normal doses), adverse drug events (an injury from a medicine or lack of an intended medicine) and medication errors (errors related to the medication use process that may or may not result in adverse drug outcomes).1 This chapter focuses on errors that occur during the medication use process, which includes the prescribing, dispensing, and administration phases of medication use; monitoring the patient for expected and unexpected outcomes; and patient compliance.
Types of Medication Errors
Medication errors can occur at any point during the medication use process. They do not occur only in the pharmacy. For example, medication errors can occur when a prescriber writes an order (during the prescribing process), when a nurse transcribes a medication order, when office personnel phone in a prescription to the pharmacy, or when patients do not take their medication as directed (i.e. patient compliance in adhering to the drug regimen).
Pharmacy technicians need to be aware of and concerned with all types of errors, not just those specifically occurring in the pharmacy. It is possible for a pharmacist to miss an error but a technician to notice it. According to the ASHP Guidelines on Preventing Medication Errors in Hospitals, medication errors can be categorized into eleven types, including:2
 Prescribing Errors
Prescribing Errors
 Omission Errors
Omission Errors
 Wrong Time Errors
Wrong Time Errors
 Unauthorized Drug Errors
Unauthorized Drug Errors
 Improper Dose Errors
Improper Dose Errors
 Wrong Dosage Form Errors
Wrong Dosage Form Errors
 Wrong Drug Preparation Errors
Wrong Drug Preparation Errors
 Wrong Administration Technique Errors
Wrong Administration Technique Errors
 Deteriorated Drug Errors
Deteriorated Drug Errors
 Monitoring Errors
Monitoring Errors
 Compliance Errors
Compliance Errors
Note that the specific category to which an error belongs is not always obvious because of the complex nature of the medication use process. Errors can occur because of multiple factors, and therefore may fit into several categories.
Prescribing Errors
A prescribing error occurs at the time a prescriber orders a drug for a specific patient. Errors can include the selection of an incorrect drug, dose, dosage form, route of administration, length of therapy, or number of doses. Other prescribing errors include inappropriate rate of administration, wrong drug concentration, and inadequate or incorrect instructions for use. When evaluating whether a medication was prescribed in error, it is important to consider patient characteristics, such as allergies, weight, age, medical indication (condition being treated), and concurrent drug therapy, among other factors. For example, a prescription for amoxicillin 250 mg PO TID may be appropriate to treat a middle ear infection in a five-year-old child, but the dose would be too high for a twelve-month-old infant and thus would be considered a prescribing error. Prescriptions that are filled incorrectly because of illegible handwriting are also considered prescribing errors.
Omission Errors
Failure to administer an ordered dose to a patient in a hospital, long-term care facility, or other facility before the next scheduled dose is considered an omission error. An omission error occurs when a dose is completely omitted as opposed to administered late. An example would be when a patient forgets to take their morning dose of acarbose (Precose). If a dose is ordered to be held for medical reasons, it is not considered an error. Examples of times when an omitted dose is not an error are when the patient cannot take anything by mouth (NPO) prior to a procedure or when health care providers are waiting for drug level results to be reported. In addition, not administering medications because a patient refuses to take them is not considered an error.
Wrong Time Errors
Timing of administration is critical to the effectiveness of some medications. Maintaining an adequate blood level of some drugs, such as antibiotics, frequently depends on evenly spaced, around-the-clock dosing. Administering doses too early or too late can affect the drug blood level and consequently the efficacy of the drug.
Long-term care facilities and hospitals frequently establish standardized administration times to maintain consistency because it could be harmful to a patient if a daily dose is administered inconsistently. It would, however, not be realistic to expect all morning doses to be administered at, for example, exactly 0800; therefore, an acceptable interval surrounding the scheduled time is usually established. An institution may determine that administering medications within thirty minutes of the scheduled time (thirty minutes before or after) is acceptable. Medications administered outside this window would be considered wrong time errors. For example, if an order were written for cefazolin 1g IV to be given within thirty minutes of the beginning of surgery, but the dose is given sixty minutes prior to surgery, a wrong time error would occur. Wrong time errors are occasionally unavoidable because the patient is away from the patient care area for a test or the medication is not available at the time it is due.
Unauthorized Drug Errors
Administration of a medication to a patient without proper authorization by the prescriber is categorized as an unauthorized drug error. An unauthorized drug error might occur if a medication for one patient was given mistakenly to another patient or if a nurse gave a medication without a prescriber order. Another cause is when patients “share” prescriptions at home. In some states, refilling a prescription that has no refills remaining without authorization from the prescriber is another example of an unauthorized drug error.
Some health care facilities establish guidelines or protocols that allow flexibility in administering medications on the basis of specific patient parameters. For example, a post-surgical protocol may allow a nurse to administer an infusion of potassium chloride when a patient’s blood potassium level falls below a specified level. The dose of potassium chloride may vary depending on how low the blood level is. Administration of a medication outside established guidelines is another example of an unauthorized drug error.
Improper Dose Errors
Improper dose errors occur when a patient is given a dose that is greater or less than the prescribed dose. This type of error can occur when there is a delay in documenting a dose, or absence of documentation, that results in an additional dose being administered. Inaccurate measurement of an oral liquid is also an improper dose error. Excluded from this category are doses that cannot be accurately measured or are not specified, as in topical applications. Variances that occur from apothecary to metric conversions are excluded as well.
Wrong Dosage Form Errors
Doses administered or dispensed in a different form than ordered by the prescriber are classified as wrong dosage form errors. Depending on state laws and health care facility guidelines, dosage form changes may be acceptable to accommodate particular patient needs. For example, dispensing a liquid formulation without a specific prescription to a patient who has difficulty swallowing tablets might be an acceptable dosage form change.
Wrong Drug Preparation Errors
Drugs that require reconstitution (adding liquid to dissolve a powdered drug), dilution, or special preparation prior to dispensing or administration are subject to wrong drug preparation errors. Examples include reconstituting an azithromycin oral suspension with an incorrect volume of water, using bacteriostatic saline instead of sterile water to reconstitute a lyophilized powder for injection, and not activating an ADD-Vantage® IV admixture bag. Using the wrong base product when compounding an ointment is another example of a wrong drug preparation error.
Wrong Administration Technique Errors
Doses that are administered using an inappropriate procedure or incorrect technique are categorized as wrong administration technique errors. For example, a subcutaneous injection that is given too deep and an intravenous (IV) drug that is allowed to infuse via gravity instead of using an IV pump are classified in this category. Instilling eye drops in the wrong eye is another example of an error in this category.
Deteriorated Drug Errors
Although sometimes cumbersome, monitoring expiration dates of products is very important. Drugs used past their expiration date may have lost potency and may be less effective or ineffective. Refrigerated drugs stored at room temperature may decompose to the point at which their efficacy is less than optimal. Medications that are dispensed or administered beyond their expiration date and medications that have deteriorated because of improper storage are considered to be deteriorated drug errors.
Monitoring Errors
Monitoring errors result from inadequate drug therapy review. For example, ordering serum drug levels for a patient taking phenytoin to prevent seizures, but not reviewing them or not responding to a level outside of the therapeutic range is a monitoring error. Not ordering drug levels when required or prescribing an antihypertensive agent, which lowers blood pressure, and failing to check blood pressure are monitoring errors as well.
Compliance Errors
Medication errors are committed by patients when they fail to follow or adhere to a prescribed drug regimen. This is referred to as a compliance error. Compliance errors can be detected when a patient requests refills for prescriptions at unreasonable intervals (too long after or too soon before a refill is due) without a reasonable explanation. An example of a compliance error is when a patient taking antibiotics to treat an infection discontinues therapy before all the doses are taken.
Other Errors
Errors that cannot be placed into one of the preceding eleven categories are grouped together in a miscellaneous category. A medication that is dispensed without adequate patient education might be considered an error in this category. For example, a prescription for alendronate (Fosamax) that is dispensed without informing the patient (either verbally, by the use of an auxiliary label, or by the distribution of a medication teaching guide) that they must not lie down for a minimum of thirty minutes after taking their dose would be considered a miscellaneous error.
 Some of the errors as defined in the ASHP guidelines seem to apply primarily to patients in health care facilities. These same definitions, however, can be applied to home health care, clinic, and physician office settings, as well as the outpatient pharmacy practice settings.
Some of the errors as defined in the ASHP guidelines seem to apply primarily to patients in health care facilities. These same definitions, however, can be applied to home health care, clinic, and physician office settings, as well as the outpatient pharmacy practice settings.
Incidence
Although medication errors are common, determining their actual numbers is difficult. Few studies provide a complete and thorough evaluation of errors within the entire medication use process. It is difficult to project data from studies on medication errors because of the different methods used to detect errors and the various definitions of errors. In addition, the focus of some studies is on just physician, nursing, or pharmacy errors or just one component of the medication use process.
Medication errors can occur at any point in the medication use process. Millions of doses are administered daily in health care facilities and patient homes, and the volume of prescriptions filled annually in communitybased pharmacies, including mail order, is approximately 3.54 billion.3 On the basis of these estimates alone, it is apparent that even with a high rate of accuracy, a small percentage of errors can result in a large number of medication errors. In addition, the number of new drugs and dosage forms available continues to grow, making it difficult to keep up with new developments in pharmacy. Staying abreast of technological advances and complex medication regimens requires professional commitment.
 Medication error awareness and prevention must be a high priority in all health care facilities and pharmacies.
Medication error awareness and prevention must be a high priority in all health care facilities and pharmacies.
Medication Error Rates
This section describes medication error rates reported in some studies. It provides an overview of the complexity of studying medication errors owing to the different monitoring, measuring, and reporting techniques used. It also reviews differences in the studies that contribute to varying medication error rates reported in the literature.
The Harvard medical practice study that analyzed the incidence of adverse events in hospitalized patients found that 19% of the adverse events that occurred in hospitalized patients were related to drug complications.4This study demonstrates that complications from drugs, including those caused by errors, are a significant cause of medical management injuries in hospitalized patients.
Physician prescribing error rates in hospital and community settings have been reported to be 0.3 to 1.9%.5–7 One study determined that almost one-third (28.3%) of the prescribing errors were potentially harmful if not followed up by a pharmacist.6 An evaluation of causes of prescribing errors in hospitals found that the majority of potentially serious prescribing errors were made because of performance lapses (knowing the right thing to do, but accidentally doing something else) by the physician or because of failure to adhere to established procedures.8 Further, it has been observed that errors occurring earlier in the medication use process (in the prescribing phase) are more likely to be detected and corrected than those occurring later in the process (in administration).9
Physician prescribing is only the first step in the medication use process. Other studies have evaluated medication errors occurring at other stages. Error rates of pharmacists dispensing in the outpatient setting have been reported to be approximately 12%.10,11 There are conflicting data evaluating the relationship between the number of serious errors and the number of prescriptions filled.10,11 The medication error rate in hospitals has been estimated to be one error per patient per day.12 One study evaluated the number of errors occurring in the drug administration phase in thirty-six hospitals and skilled nursing facilities and found that 19% of all doses were not administered correctly. The majority (43%) of errors were due to wrong time of administration.13 The Institute of Medicine estimates that approximately 1.5 million people are harmed by medications each year. As many as 400,000 of these adverse events are considered to be preventable.14
Medication error studies report different error rates. Pharmacy technicians should recognize that the differences in error rates may be due to differences in how studies were performed, the various techniques and definitions used, and the scope of the study. Many errors are identified and corrected before medications reach the patient and these errors might not be accounted for. Studies also show that a small percentage of errors lead to adverse events in patients.15
Medication Error Reporting
The rate of medication errors is often based on incident reports. Ideally, health care providers complete incident reports when a medication error is discovered. That does not always happen, however, because many health care personnel lack the knowledge to identify errors, lack the time to document them, or are afraid of negative consequences.
Many times errors are discovered when a pharmacist checks a prescription or medication order prior to dispensing, and the error is corrected promptly before the medication reaches the patient. Often, the error is not documented because it is not recognized as an error or the reporting process is cumbersome. For these reasons, the number of medication errors is probably higher than reported.
Reporting medication errors can sometimes be a frightening experience. Health care personnel may be afraid of disciplinary or punitive actions or of the backlash of reporting an error made by a coworker. They may also be concerned about liability issues should a negative outcome occur because of an error.
It is apparent that medication errors occur every day in all practice settings. Fortunately, most of these errors are detected and corrected before the medication ever reaches the patient. Some medication errors do, however, reach the patient, and some errors result in negative outcomes.
Impact of Medication Errors
The outcomes of medication errors range from no effect to minor discomfort to devastating long-term disability or death.16,17 Often, predicting the outcome and significance of a medication error is difficult because so many factors are involved. Such factors include the type of medication error, the health status of the patient, the pharmacologic classification of the drug involved, the route of drug administration, the timing of drug administration, the cost to the health care system, and the damage to the patient’s trust in care providers.
Impact on the Patient
In a report of five pediatric patients who received overdoses of vincristine (a chemotherapy drug), three patients died and two recovered.18 Of the three patients who died, two received a tenfold overdose, and the third was very ill with advanced stage leukemia. The two children who recovered were in remission (i.e., their leukemia was under control) at the time and received smaller overdoses. In this situation, the health status of the patients and magnitude of the overdose helped determine the significance of the error.
Sometimes, not receiving a drug or receiving it late can harm patients as well. Administration of a dose of phenytoin to prevent seizures was delayed twenty-eight hours in an elderly patient, resulting in the patient experiencing a seizure.19 The patient subsequently underwent extensive surgery to repair a jaw fracture that resulted from a fall during the seizure. These events can be attributed to one medication error—late administration of the phenytoin. Many case reports describe adverse drug events caused by medication errors.
Financial Implications
Not only can medication errors lead to negative patient outcomes, they can prolong hospital stays and increase health care expenses.20 Treating adverse events is estimated to cost billions of dollars annually.14,16,21,22 It was estimated that $1.5 million was spent in a single year to treat adverse drug events at one hospital.21 Another study evaluated the cost of drug-related patient injury and death in the ambulatory setting. That study estimated that the United States spends $76.6 billion annually to manage those drug-related occurrences, some of which were due to medication errors.22 Not only must the cost of additional medical management be considered, but also the legal fees and out-of-court settlements resulting from malpractice claims.
Medication errors or the use of contaminated drugs that result in patient disability or death are categorized as “never events” by the National Quality Forum, a nonprofit organization represented by numerous health care, consumer advocate, and philanthropic groups. Some states have revised Medicaid reimbursement to include provisions that will not reimburse hospital expenses associated with treating conditions that result from never events.23
Loss of Trust
Patients can lose faith in the medical community as a result of experiencing or reading about an adverse drug event. They may choose to switch pharmacies or physicians or even hesitate to seek medical help for fear of not receiving quality care. Patients may also seek nonconventional treatments from outside the medical community. Personnel responsible for medication errors that result in significant patient injury also can lose confidence in themselves as practitioners.
Fortunately, most medication errors are detected and corrected before the medication is dispensed to the patient or the patient care area. Medication errors do occur, however, and can result in reversible or permanent negative patient outcomes. They can also be associated with a financial impact to an individual, an institution, and the overall health care system.
Causes of Medication Errors
Medication errors can be attributed to a number of different causes. It would be unfair to place blame solely on an individual without considering factors that can contribute to an error. Administrators of health systems constantly strive to decrease the presence of factors in the medication use system that contribute to medication errors. In turn, each health care worker must also strive to minimize the occurrence of medication errors. One of the best ways to do this is to become familiar with the most common causes of medication errors. Medication errors are most often attributed to one or more of the following: calculation errors, improper use of zeros and decimal points, inappropriate use of abbreviations, careless prescribing, illegible handwriting, missing information, drug product characteristics, compounding/drug preparation errors, prescription labeling, work environment and personnel issues, and deficiencies in medication use systems.
Calculation Errors
Reports show that numerous medication errors are caused by errors in mathematical calculations. Miscalculation of doses can lead to serious patient harm or even death.17,24 Calculation errors are made by prescribers, pharmacists checking doses for appropriateness or calculating doses, technicians compounding products, and nurses preparing or administering doses. Even with the use of calculators and computers, health care personnel frequently make calculation errors.25
The pediatric population is particularly at risk for calculation errors. It is not uncommon for pediatric doses to be determined by the patient’s weight, requiring an interim step to calculate the final dose. Many drugs are not available in pediatric formulations, so adult formulations must be diluted or manipulated multiple times to get the appropriate dose.
Personnel with multiple years of experience are just as likely to make mathematical errors as inexperienced personnel.26,27 Calculation errors are often made by using the wrong concentration of stock solutions, misplacing a decimal point, or using wrong conversions. Personnel also neglect to double-check their work, or rely on their memory instead of looking up a conversion. In some cases, they fail to ask themselves, “Does the answer seem reasonable?”
 Always double-check calculations and look up conversions instead of relying on memory. Think about whether the answer makes sense.
Always double-check calculations and look up conversions instead of relying on memory. Think about whether the answer makes sense.
Another way to decrease the risk of a calculation error is to ask a pharmacist or another technician to double-check the calculation prior to preparing the product. The calculation should be performed independently and should be compared with the original answer. This system is an effective way to prevent calculation errors. (See Chapter 14, Pharmacy Calculations.)
Decimal Points and Zeros
Misplacing a decimal point by one place results in errors tenfold greater than or less than intended. For drugs with a narrow therapeutic range (e.g., digoxin, phenytoin, warfarin, gentamicin), the consequences can be significant.
Decimal point errors can occur as a result of a miscalculation, as described above, and also when writing orders or instructions. Failure to write a leading zero in front of a number less than one (e.g., .1 mg instead of 0.1 mg) can result in the number being read as a whole number (1 mg). Writing unnecessary trailing zeros can also be confusing (e.g., 10.0 mg instead of 10 mg, which could be misinterpreted as 100 mg). Medication order sheets with lines can sometimes cause a decimal point to be overlooked on the copy that is sent to the pharmacy. Medication orders that are received via fax should be reviewed carefully since artifact (insignificant markings on the page) might cause the order to be misinterpreted.
When writing numbers, a leading zero should always be used with a decimal point for numbers less than one (0.1 mg, not .1 mg) and a decimal point and trailing zero should never be used for whole numbers (10 mg, not 10.0 mg). Technicians must be aware of the potential for decimal point errors due to misplaced or missing decimal points when interpreting orders, and questionable orders should be brought to the attention of the pharmacist.
RX for Success
A good general rule to follow is to question any dose that requires less than one-half or more than two of the dosage unit (tablet, capsule, teaspoonful). This rule often helps catch calculation errors.
Abbreviations
The abbreviation of medical terms and drug names can lead to medication errors. For example, the use of the abbreviation “AZT” for zidovudine (Retrovir), an antiretroviral agent used to treat HIV infection, could also be interpreted as azathioprine (Imuran), an immunosuppressant agent, which would cause harm to the patient if an error was made.
Another example of an abbreviation error is the use of “U” as an abbreviation for units. This abbreviation could result in a tenfold error if the “U” were read as a “zero” (e.g., 10 U insulin could be read as 100 insulin). A daily order written as “QD” instead of “daily” may be troublesome because it could be read as “QID” (four times a day) or “OD” (every other day).
The Joint Commission has developed a list of dangerous abbreviations and dose designations that should not be used (table 17-1).28 This “do not use” list applies to physician orders and medication documentation that is handwritten or printed on preprinted order forms. The Institute for Safe Medication Practices (ISMP) has a more extensive list of abbreviations and terms that have been misinterpreted and led to medication errors. This list is available on their website.29
Table 17–1. The Joint Commission Official “Do Not Use” List
| Do Not Use | Potential Problem | Use Instead |
| U (unit) | Mistaken for “0” (zero), the number “4” (four) or “cc” | Write “unit” |
| IU (International Unit) | Mistaken for IV (intravenous) or the number 10 (ten) | Write “International Unit” |
| Q.D., QD, q.d., qd (daily) | Mistaken for each other | Write “daily” |
| Q.O.D., QOD, q.o.d, qod (every other day) | Period after the Q mistaken for “I” and the “O” mistaken for “I” | Write “every other day” |
| Trailing zero (X.0 mg)* Lack of leading zero (.X mg) | Decimal point is missed | Write X mg Write 0.X mg |
| MS | Can mean morphine sulfate or magnesium sulfate | Write “morphine sulfate” Write “ magnesium sulfate” |
| MSO4 and MgSO4 | Confused for one another |
1 Applies to all orders and all medication-related documentation that is handwritten (including free-text computer entry) or on preprinted forms.
*Exception: A “trailing zero” may be used only where required to demonstrate the level of precision of the value being reported, such as for laboratory results, imaging studies that report size of lesions, or catheter/tube sizes. It may not be used in medication orders or other medication-related documentation.
Additional Abbreviations, Acronyms and Symbols (For possible future inclusion in the Official “Do Not Use” List) | ||
| Do Not Use | Potential Problem | Use Instead |
| > (greater than) | Misinterpreted as the number | Write “greater than” |
| < (less than) | “7” (seven) or the letter “L” Confused for one another | Write ”less than” |
| Abbreviations for drug names | Misinterpreted due to similar abbreviations for multiple drugs | Write drug names in full |
| Apothecary units | Unfamiliar to many practitioners Confused with metric units | Use metric units |
| @ | Mistaken for the number “2” (two) | Write “at” |
| cc | Mistaken for U (units) when poorly written | Write “mL” or “ml” or “milliliters” (mL is preferred) |
| µg | Mistaken for mg (milligrams) resulting in one thousand-fold overdose | Write “mcg” or “micrograms” |
There are many accepted abbreviations in health care, and use of abbreviations can be efficient if everyone understands and agrees on the definitions. The Joint Commission’s 2009 National Patient Safety Goals include a recommendation that organizations create a list of abbreviations, acronyms, and symbols that should not be used.30 This list should include the terms on the official “do not use” list and any additional terms that the organization feels might contribute to medication errors. Being unaware of the accepted interpretation of abbreviations can lead to errors, and creating new abbreviations that others may not understand should be avoided.31 ASHP recommends that an approved list of abbreviations be developed by the organization’s Pharmacy and Therapeutics Committee or its equivalent.2 Abbreviations not on the approved list should be reviewed carefully before processing an order.
RX for Success
Write out directions for medications rather than using nonstandard or ambiguous abbreviations. The complete drug name, preferably the generic, should be used.
Technicians should become familiar with the list of abbreviations approved at their facility. Community pharmacies generally do not have a formal, approved list of abbreviations. Posting a list of commonly accepted medical abbreviations in the pharmacy, however, is beneficial. Technicians should also be familiar with the abbreviations and dose designations that are considered dangerous and pay particular attention to them when filling orders. See Appendix A at the end of this book for a list of medical abbreviations.
High Alert Medications
Several medications or drug classes have been categorized as high alert medications because of their high risk of causing serious harm to patients when given in error. Errors with drugs designated as high alert do not necessarily occur more frequently than others. According to the ISMP, the following medications are examples of high alert medications:32
1. heparin
2. narcotics and opiates (e.g., morphine, hydromorphone, oxycodone)
3. potassium chloride injection
4. insulin
5. chemotherapeutic agents (e.g., methotrexate, vincristine, doxorubicin)
6. neuromuscular blocking agents (e.g., vecuronium, cisatracurium, succinylcholine)
A complete list of high alert medications and drug classes can be found on the ISMP website.32
Pharmacies should evaluate how high alert medications are handled and institute specific error reduction strategies as appropriate. Such strategies might include limiting the number of strengths or vial sizes of medications stocked, use of special auxiliary labeling, strategic storage locations, double-checks, and the use of standardized or preprinted orders.
RX for Success
Become familiar with medications designated as “high alert” in your organization (e.g., heparin, fentanyl, paralyzing agents) and make sure to follow any extra precautions that have been established specifically for these products.
Prescribing Issues
Medication errors can result from the way a drug is prescribed. Issues associated with the prescribing component of the medication use processes that may contribute to an error include verbal orders, confusion regarding the concentration of a product, illegible handwriting, missing information, use of the apothecary system, and writing doses based on the course of therapy as opposed to a daily dose. This section describes how these prescribing issues can lead to errors, in addition to ways to minimize such errors.
Verbal and Telephone Orders Oral orders (orders given orally by a prescriber) can lead to medication errors when they are heard incorrectly or when they are transcribed to writing or entered into a computer incorrectly.33 This can occur when an order is orally communicated in a face-to-face situation (verbal order) or when an order is given over the telephone (telephone order). The use of cellular phones and poor quality connections can make telephone orders even more difficult to understand. With the number of similar sounding products available, it is easy to misunderstand an oral order. In one case report, a telephone order from a physician for Prograf (tacrolimus), an immunosuppressant, 10 mg daily was misunderstood by the pharmacist as Prozac (fluoxetine), an antidepressant. As the pharmacist was counseling the patient on the use of fluoxetine, it became apparent that there had been a mix-up.34
Verbal or telephone orders should only be used in situations when it is impossible or impractical for the prescriber to write the order or submit it electronically. A written copy of the order should be placed in the pharmacy’s prescription file or the patient’s medical record. Institutional pharmacies routinely require the prescriber to confirm verbal or telephone orders by signature. The use of oral orders should particularly be avoided in chemotherapy prescribing because of the complexity of these orders and the potentially lethal impact of mistakes with these drugs.
 The recipient of a verbal or telephone order should immediately write down the order and read it back to the prescriber to ensure clarity of the order.
The recipient of a verbal or telephone order should immediately write down the order and read it back to the prescriber to ensure clarity of the order.
Although cell phones, unfamiliar terminology, accents, and poor connections can make taking an oral order difficult, it is the responsibility of the technician to ask the other party to clarify parts of the order that are not clear. Simply asking the other party to spell the names of the medications and other words that are unclear and repeating the order back to the other party can help ensure that the right order is received. When reading back numbers that may be easily misunderstood, it is helpful to say each numeral. For example, 50 should be stated as “fifty as in five zero.”
 Many states limit the acceptance of verbal medication orders to registered pharmacists. State law and pharmacy policy should be consulted to determine what role, if any, technicians may play in accepting verbal orders.
Many states limit the acceptance of verbal medication orders to registered pharmacists. State law and pharmacy policy should be consulted to determine what role, if any, technicians may play in accepting verbal orders.
Drug Concentration. Failure to include the concentration of a liquid formulation in a prescription can result in a wrong dose being dispensed. For example, an order for amoxicillin suspension 1/2 tsp (2.5 mL) TID does not specify the concentration of the suspension, causing confusion as to the actual dose ordered. It could be interpreted as 62.5 mg (1/2 tsp of 125 mg/5 mL) or 125 mg (1/2 tsp of 250 mg/5 mL).
Writing “1 amp,” “1 vial,” or “1 cap” can lead to errors when products come in multiple strengths, doses, or vial sizes. An order for one “vial” of magnesium sulfate might be filled with a 2 mL vial (8 mEq), a 20 mL vial (16 mEq), or a 10 mL vial of 50% concentration (40 mEq). The pharmacist should clarify ambiguous doses before the technician processes the order.
Illegible Handwriting. The poor handwriting of physicians frequently is the subject of jokes; however, illegible handwriting of any health care provider is no laughing matter when it contributes to medication errors. With the many sound-alike and look-alike drug names on the market, it is easy to understand how illegible handwriting can lead to errors. One report describes a poorly written order for Aredia (pamidronate), a blood calcium lowering agent, 60 mg IV that was filled and administered as Adria, a commonly spoken term for Adriamycin (doxorubicin), a chemotherapy agent. The patient received approximately 20% of the dose before the error was noticed and experienced bone marrow toxicity (decrease in blood cell counts) as a consequence.35 Both agents are reasonable medications for a cancer patient and are prescribed in doses of 60 mg, but the poorly written medication name led to the mix-up. Facsimile transmission of handwritten orders can further complicate interpretation of illegible handwriting.
The entire order should be carefully evaluated when trying to decipher illegible handwriting. Sometimes the dose or route of administration is helpful in determining what medication was ordered. Assistance should be obtained from a pharmacist when orders are difficult to interpret because of illegible handwriting. The pharmacist should contact the prescriber to clarify orders that are difficult to interpret. In some practice settings, technicians may play a role in obtaining order clarifications.
Using standardized, preprinted order forms for complex drug regimens is one way to minimize illegible handwriting.2 Computer generated and typewritten labels reduce medication errors by making the medication labels easier to read for both health care personnel and patients. The use of upperand lowercase lettering (as opposed to all uppercase) also improves readability.
Missing Information. Lack of complete medical information about the patient, such as age, weight, height, allergies, and diagnosis, can contribute to medication errors. Medical information is important because dose often depends on the indication and severity of the condition. Unfortunately, physicians do not routinely write the indication on prescriptions, and patients do not always fully understand their conditions. In some hospital pharmacies, medical information is available only in the chart because the pharmacy computer system does not interface with the main hospital computer.
Thorough and complete medication profiles should be maintained for all patients. These profiles should include at a minimum, current prescription and nonprescription medications, allergies, age, height, and weight of the patient. Previous medication use is also helpful. Profiles should be kept current and referred to routinely. It may be necessary to question the patient or contact the prescriber to obtain this information, because pharmacists often need it to check for appropriateness. See Chapter 13, Processing Medication Orders and Prescriptions, for additional information on this topic.
Apothecary System. The apothecary system is an outdated system of measurement that some prescribers continue to use. This system can lead to errors because it is unfamiliar to many health care personnel and must be converted to the metric system. The fact that “1 gr” (grain) may be interpreted as 60 mg or 65 mg is confusing enough, but if it is written sloppily, it could be misread as “1 gm” (1 gram = 1000 mg). Prescribers should be discouraged from using the apothecary system.
Apothecary conversion charts should be readily available in the pharmacy, and technicians should become familiar with commonly used apothecary symbols and their metric equivalents, which are available in Chapter 14, Pharmacy Calculations.
Course Dose vs. Daily Dose. Chemotherapy medication regimens are commonly prescribed on a per course or cycle of treatment basis as opposed to a per dose basis. This practice increases the risk of medication errors because the orders are often difficult to interpret.36 Many chemotherapy treatments require a patient to receive medication over several days and then rest (receive no medication treatment) for several days or weeks. This allows time for the patient to recover from the side effects and for the medications to work in the optimal phase of the tumor cell cycle. One course of treatment may consist of several medications given on one or more days during a specified time period.
An example of a chemotherapy course dose is fluorouracil 4 g/m2 IV days one, two, three, and four. This order could be misinterpreted as 4 g/m2 of fluorouracil daily for four days—a total of 16 g/m2—or as 4 g/m2 to be divided into four daily doses (1 g/m2 daily on days one, two, three, and four). Errors such as this can result in massive overdoses, leading to prolonged illness or death.
Drug Product Characteristics
Characteristics of drug products that can contribute to medication errors include look-alike and sound-alike drug names, the use of numbers or letters as part of the drug name, product labeling, color coding, and advertising.37 Drug product problems identified by the USP are forwarded to pharmaceutical manufacturers so they can address the problems by making appropriate modifications to the drug products.
Look-Alike and Sound-Alike Drug Names. Many case reports deal with medication errors caused by confusion surrounding drug names.38–43 Hundreds of drug names either sound or look like other trade or generic drug names. The USP provides an easy to use search tool called USP’s Drug Error Finder on their Web site.44 This search tool contains over 1,400 sound-alike and lookalike medication names that have been identified in error reports. For medication errors that were reported through the USP Reporting Program, the severity of the error is also provided. ISMP also maintains a list of “confused” medication names on their website (see Appendix B).45
Sometimes errors occur because medication names look and sound similar and may even be used to treat a common condition. For example, nelfinavir (Viracept) and nevirapine (Viramune) are two antiretroviral agents used in the treatment of HIV infection. Both the brand and the generic names are similar, increasing the risk for confusion.46 Sloppy handwriting and misspelling also can contribute to drug name confusion. An order carelessly written for interferon, an immunologic agent, 1 mL was interpreted and prepared as Imferon (iron dextran) 1 mL.38 In this case, the patient’s mother questioned the dark brown coloring of the drug before it was administered and the mix-up was corrected before the patient received the wrong drug.
The likelihood of confusing two drugs with similar names is increased when the dosages of both drugs are the same. Lanoxin (digoxin) and Levoxine (levothyroxine) have similar looking and sounding names and both may be prescribed at a dose of 0.125 mg daily.40 Because of these similarities, the pharmaceutical manufacturer of Levoxine changed the trade name to Levoxyl in an effort to avoid confusion with Lanoxin.
A frequently reported mix-up occurs between quinine, an antimalarial, and quinidine, an antiarrhythmic. The names are similar, routine doses are the same, and they are frequently stocked next to each other. It is easy to see how one medication could be picked instead of the other.
Confusion with sound-alike and look-alike medication names is a growing issue with the increasing number of medication products available. In a review of medication errors associated with look-alike and sound-alike medications reported over a four-year period, the USP found that each of the top ten selling medications in the U.S. have been identified in a look-alike or sound-alike pairing.47 Pharmaceutical manufacturers are responsible for carefully selecting medication product names, keeping patient safety in mind. Approximately 30% of all new medication names reviewed by the FDA are rejected because they may lead to confusion.48 Table 17-2 lists examples of medication names that were changed to reduce the risk of prescribing errors. Health care providers can help identify potentially confusing drug names by notifying the USP or FDA with their concerns.
Numbers and Letters as Part of Medication Names. Manufacturers sometimes include numbers and letters as prefixes and suffixes to brand names (e.g., Tylenol #3, Percocet-5, Effexor-XR). Although the intent may be to indicate strength or that a product is an extended release formulation, it can lead to errors. One case of such an error reported in the literature describes an ice pack applied to the chest of a patient hospitalized with pneumonia instead of administration of the antibiotic azithromycin. The physician wrote for a “Z-Pak,” commonly prescribed this way on outpatient prescriptions. The specific dosage regimen was not written out because the product is available as a blister pack containing the entire course of therapy and is labeled with dosing instructions. The patient’s pneumonia worsened until the error was discovered two days later and appropriate treatment was initiated.49
Numbers in the medication name can be misinterpreted as the dose. For example, the prescription in figure 17-1 could be misinterpreted to take five tablets of Percocet every four hours as needed instead of one tablet of Percocet-5 every four hours as needed. A patient could become extremely drowsy or confused after taking five tablets of Percocet-5.
Letters or numbers that are omitted from brand names when writing an order can contribute to errors. For example, the immediate release form of venlafaxine (Effexor) could be dispensed instead of the extended release formulation because the “XR” part of the name was omitted in the prescription shown in figure 17-2. The extended release formulation is designed to release the drug slowly over the entire day, whereas the immediate release form releases the entire dose at once.
Table 17–2. Examples of Drug Product Names Changed to Reduce the Risk of Prescribing Errors
| Former Trade Name | Confused With | New Trade Name |
| Clonopin | Clonidine | Klonopin |
| Omacor | Amicar | Lovaza |
| Losec | Lasix | Prilosec |
| Microx | Micro-K | MyKrox |
| Reminyl | Amaryl | Razadyne |
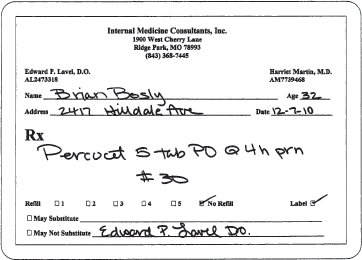
Figure 17–1. Example of a medication name that includes a number.
Product Labeling. As a marketing strategy, product labels often emphasize a manufacturer’s name or logo, potentially making it difficult to readily identify the drug name and dose. Manufacturers often use the same labeling scheme, including letter size, print, and background color, to associate the product with the manufacturer. Sometimes this strategy, which makes all labels look alike, can be detrimental.
The dosage strength and total contents of liquid formulations are not always labeled clearly. Different vial sizes of injections may be similarly labeled with the concentration (mg/mL), but too little emphasis may be placed on the total contents of the vial. When midazolam (Versed) first appeared on the market, it was available in a 5 mg/mL concentration in 1 mL and 2 mL size vials. The vial size was not prominent on either label, which made it difficult to differentiate between the 10 mg (2 mL) and 5 mg (1 mL) vials.
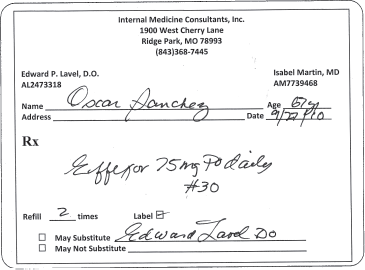
Figure 17–2. Example of a prescription for “Effexor XR” where the “XR” was omitted.
There have been numerous cases of a health care provider using a potassium chloride (KCl) injection to flush an IV line instead of normal saline because the vial sizes and labeling of the two products were similar. Manufacturers of potassium chloride injection are now required to use black vial caps and overseals with a warning that states “must be diluted.”50
Color Coding. Relying on the color of product packaging is not a safe practice. Manufacturers may change their packaging color scheme at any time, and color-coding schemes for similar products may differ among manufacturers. Sometimes there is too little difference between colors in a color scheme, which leads to mix-ups. Medication products with similar colors can be misplaced in the stock areas and could easily be dispensed in error. For example, both daunorubicin 20 mg and doxorubicin 10 mg are packaged in vials that are shaped similarly and have dark blue vial caps. They are both lyophilized powders that turn into red solutions upon reconstitution. Relying on the color of the vial cap or of the diluted solution could lead to very serious errors. The ISMP recommends that color coding medications be used cautiously because it has not been scientifically shown to prevent medication errors and several problems have been identified by its use.51
Advertising. Many practices that contribute to medication errors are perpetuated through pharmaceutical product advertising. Journal advertisements may include abbreviations, lack of adequate dosage strength identification, inappropriate use of decimal points, and so forth. The more frequently this information is seen, the more readily it is accepted by health care personnel—and the more likely it can lead to errors. These issues must be kept in mind when reviewing drug literature.
A problematic trend in marketing is the use of a brand name for products that contain different active ingredients. Manufacturers sometimes use the name of an established product to attract the public to a new product. For example, Zyrtec oral products contain the active ingredient cetirizine; however, Zyrtec Itchy Eye Drops contain the active ingredient ketotifen. Similarly, Claritin oral products contain loratadine as the active ingredient and Claritin Eye contains ketotifen. Another example of a manufacturer’s attempt to draw upon the use of a familiar brand name is Pepcid versus Pepcid Complete. Pepcid contains the active ingredient famotidine, but Pepcid Complete is a combination product containing famotidine, calcium carbonate, and magnesium hydroxide. These examples demonstrate the importance of carefully reading product labels.
Compounding/Drug Preparation Errors
Errors can occur during the compounding and drug preparation phase. These errors can be difficult for others to catch, so it is essential that technicians take steps to decrease the risk of error when compounding and preparing drug products. Such an approach includes reading the product labels carefully, not processing more than one prescription at a time, labeling prescriptions properly, storing drugs properly, maintaining a safe work environment, and keeping up with changes in the medical profession.
Reading the Label. Reading drug product labels carefully when filling an order is a step that should not be neglected. This step is extremely important because of the number of product names that sound and look alike, have similar product packaging, small print, and color coding.
RX for Success
Read the label at least three times to help prevent medication errors: when you remove it from the shelf, as it is being prepared, and as the finished product is set aside for the pharmacist to check.



