46
CHAPTER OUTLINE
This chapter reviews the treatment of acute intoxication and withdrawal states associated with the use of stimulants such as cocaine and methamphetamine (including their smokable forms “crack” and “ice”); hallucinogens such as lysergic acid diethylamide (LSD); marijuana; dissociative anesthetics such as phencyclidine (PCP), ketamine, and dextromethorphan (DXM); “club drugs” such as 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) and gamma-hydroxybutyrate (GHB); and common herbal drugs of abuse. It also reviews the treatment of the serotonin syndrome and withdrawal from multiple drugs. Psychiatric and medical complications are considered separately because they often are treated with different modalities and in different settings (e.g., in psychiatric vs. medical emergency departments). Not all of the substances reviewed here have clinically distinct intoxication or withdrawal syndromes or are there pharmacologic treatments for all such syndromes.
Successful treatment of acute intoxication, overdose, or withdrawal can facilitate entry into addiction treatment by reducing uncomfortable withdrawal symptoms that negatively reinforce drug taking. Even when successful, these early stages of treatment often are followed by relapse to substance use, with patients potentially reentering a “revolving door” of repeated detoxification programs. Short-term treatment of acute intoxication or withdrawal does not obviate the need for long-term treatment of addiction.
Pharmacologic treatment of drug intoxication and overdose generally follows one of the three approaches: increased clearance of drug from the body, either by increasing catabolism or by increasing excretion, or both (1); blockade of the neuronal site to which the drug binds to exert its effect (as through the use of naloxone to block the muopioid receptor in the treatment of opiate overdose); and counteracting effects of the drug through alternative neuropharmacologic action.
Pharmacologic treatment of any drug withdrawal syndrome generally follows one of the two approaches: suppression by a cross-tolerant medication from the same pharmacologic class—usually a longer-acting one to provide a milder, controlled withdrawal (as in the use of the opioid methadone for opiate detoxification)—and/or reducing the signs and symptoms of withdrawal by targeting the neurochemical or receptor systems that mediate withdrawal (as in the use of the nonopiate clonidine to treat opiate withdrawal).
The application of these pharmacologic treatment approaches to the drugs reviewed in this chapter is limited. There may be no practical method for altering drug clearance (as with marijuana), or no specific drug receptor sites may have been identified. Even when a receptor site has been identified, there may not be a clinically useful antagonist. Finally, current understanding of the neuropharmacologic processes that mediate intoxication or withdrawal may be too limited to suggest appropriate pharmacologic interventions. Thus, clinical stabilization, supportive management, and palliation of symptoms often remain the mainstays of treatment.
STIMULANTS
Stimulant Intoxication
The acute psychological and medical effects of cocaine, amphetamines, and other stimulants are attributable principally to increases in catecholamine neurotransmitter activity. Enhanced catecholamine activity occurs through blockade of the presynaptic neurotransmitter reuptake pumps (as by cocaine) and by presynaptic release of catecholamines (as by amphetamines) (2). Resulting stimulation of the corticomesolimbic dopamine brain reward circuit mediates the desired (and addicting) psychological effects of stimulants. The resulting stimulation of the sympathetic nervous system leads to peripheral vasoconstriction (with organ ischemia), increased heart rate, and lowered seizure threshold, among other adverse effects. Table 46-1 lists acute medical complications of stimulant intoxication.
TABLE 46-1 ACUTE MEDICAL COMPLICATIONS OF STIMULANT INTOXICATION
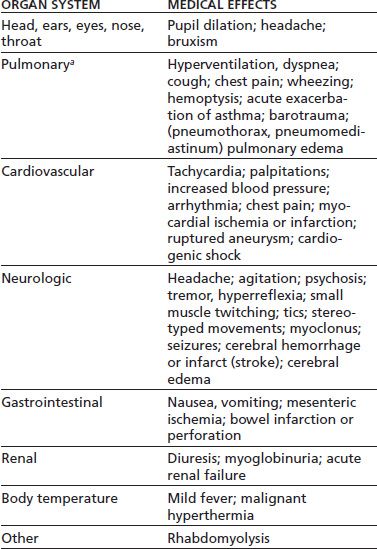
aAll pulmonary complications except hyperventilation and pulmonary edema come primarily from the smoked route of administration.
Sources: Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart 2000;83:627–633; Neiman J, Haapaniemi HM, Hillblom M. Neurological complications of drug abuse: pathophysiological mechanisms. Eur J Neurol 2000;7:595–606; Schuckit MA. Drug and alcohol abuse. A clinical guide to diagnosis and treatment, 6th ed. New York: Springer, 2006; Tashkin DP. Airway effects of marijuana, cocaine, and other inhaled illicit agents. Curr Opin Pulm Med 2001;7:43–61. Refs. (3–6).
Blockade of presynaptic catecholamine reuptake sites or postsynaptic receptors should, in principle, be an effective treatment for stimulant intoxication. Several medications have shown promise in attenuating the acute subjective effects of stimulants, such as bupropion (7), aripiprazole (8), risperidone (9), topiramate (10), and modafinil (11). Another method of attenuating the effects of stimulant intoxication might be to decrease drug availability in the central nervous system (CNS) by binding it peripherally with antidrug antibodies or by increasing its catabolism (1). The latter approach could be implemented with catalytic antibodies or with the endogenous cocaine-metabolizing enzyme butyrylcholinesterase (BChE, E.C. 3.1.1.8) or other esterases.
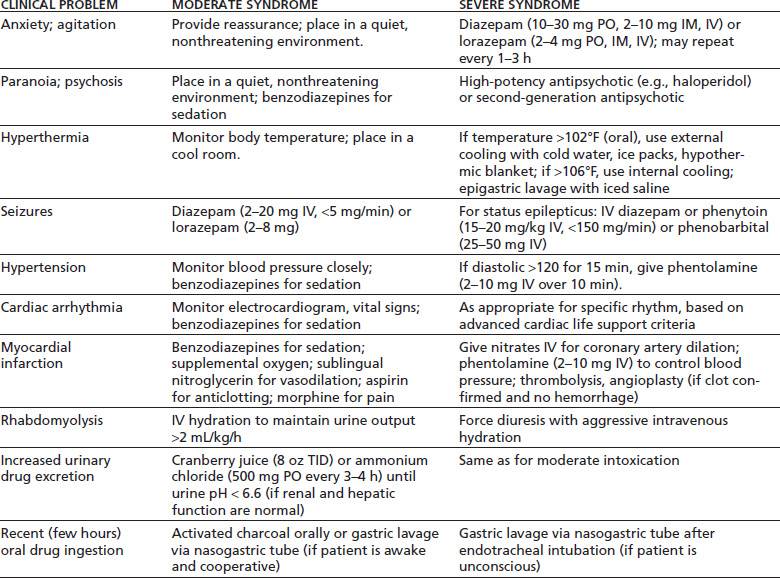
Table 46-2 gives an overview of treatment for the acute psychiatric and medical complications of stimulant intoxication.
TABLE 46-2 TREATMENT OF ACUTE STIMULANT INTOXICATION
Psychological and Behavioral Effects of Stimulant Intoxication
The initial effects of stimulant intoxication include increased energy, alertness, and sociability; elation; euphoria; and decreased fatigue, need for sleep, and appetite (12,13). At this stage, users do not seek or need treatment. With high-dose or repeated use, stimulant intoxication usually progresses to unwanted effects such as anxiety, irritability, interpersonal sensitivity, hypervigilance, suspiciousness, grandiosity, impaired judgment, stereotyped behavior, and psychotic symptoms such as paranoia and hallucinations. Up to three-quarters of stimulant users report paranoia or psychotic symptoms associated with their use, although the contribution of acute versus chronic stimulant exposure is often unclear (14–16). These results may also reflect selection bias among users who come to medical or research attention. Stimulant users typically remain alert and oriented, but the delusional state may impair judgment, cognition, and attention.
Patients with stimulant-induced psychoses may closely resemble those with acute schizophrenia and may be misdiagnosed as such (14,17). Cocaine-induced psychosis may differ from acute schizophrenic psychosis in having less thought disorder and bizarre delusions and fewer negative symptoms such as alogia and inattention (14). Stimulant-induced hallucinations may be auditory, visual, or somatosensory (14,18). Tactile hallucinations are especially typical of stimulant psychosis, such as the sensation of something crawling under the skin (formication). Specific genetic variations may account for some differences in individual vulnerability to stimulant-induced psychosis (19). Panic reactions are common and may evolve into a panic disorder (5). This may be exacerbated by anxiety elicited by the physiologic symptoms commonly associated with stimulant use, such as palpitations and hyperventilation.
Very severe stimulant intoxication may produce an excited delirium or organic brain syndrome that can be fatal (20,21). Patients should be evaluated promptly for an acute neurologic lesion (e.g., intracranial bleeding) or a preexisting neuropsychiatric condition and be treated aggressively (22).
Management of Psychological and Behavioral Effects of Stimulant Intoxication
The initial clinical evaluation should include a drug use history and drug toxicology to confirm stimulant intoxication. As the patient’s condition permits, further evaluation should rule out other potential medical (hyperthyroidism, hypoglycemia) or neuropsychiatric (panic or bipolar affective disorder) conditions (23). The initial treatment approach is nonpharmacologic (24,25). The patient should be observed in a quiet environment with minimal sensory stimulation to avoid exacerbating symptoms. Treatment staff should interact in a calm and confident manner, using the “ART” approach developed at the Haight-Ashbury Free Clinic in San Francisco: acceptance of the patient’s immediate needs (such as pain relief or use of the bathroom) and reassurance that the condition is due to the drug and likely will dissipate within a few hours and “talk down” to provide reality orientation and avoid hostility. All procedures should be explained to the patient before initiation. Physical restraints to control agitation or violent behavior should be avoided unless absolutely necessary. The use of restraints can increase the risk of hyperthermia and rhabdomyolysis, with resulting severe medical complications (26,27).
If medication is needed, most experts prefer benzodiazepines (such as diazepam [10 to 30 mg PO or 2 to 10 mg IM or IV] or lorazepam [2 to 4 mg PO or 1 to 2 IM or IV]) over antipsychotics to control severe agitation, anxiety, or psychotic symptoms (5,28,29), although there are very few controlled clinical trials (27). Benzodiazepines protect against the CNS and cardiovascular toxicities of stimulants, whereas antipsychotics may worsen the sympathomimetic and cardiovascular effects, lower the seizure threshold, increase the risk of hyperthermia, or precipitate extrapyramidal reactions (28). Parenteral benzodiazepine dosing may be repeated every 5 to 10 minutes until light sedation is achieved. If an antipsychotic is needed to control psychosis, a high-potency agent such as droperidol, haloperidol (5 to 10 mg PO, IM, or IV), or risperidone (2 to 4 mg PO) is preferred because of its minimal anticholinergic activity. Anticholinergic activity should be avoided because it may contribute to delirium and hyperthermia (by impairing heat dissipation from sweating). Chlorpromazine (30) and haloperidol (31) have been used safely in treating children with severe amphetamine poisoning. There is little evidence to guide treatment with second-generation antipsychotics, such as ziprasidone, risperidone, quetiapine, and olanzapine. Aripiprazole has been suggested as a promising medication because of its partial dopamine agonism (32).
A psychotic or agitated patient who has not responded to initial treatment should be hospitalized until the episode has resolved. This usually occurs within a few days if no more stimulants are ingested (5). Psychiatric symptoms that persist beyond a few days suggest an etiology other than stimulant use (24,33,34). Transient psychotic symptoms during periods of abstinence (“flashbacks”) have been reported among methamphetamine users (35).
Medical Effects of Stimulant Intoxication
Mild stimulant intoxication (the state desired by users) may be accompanied by one or more self-limiting physiologic effects such as restlessness, sinus tachycardia, hyperventilation, mydriasis, bruxism, headache, diaphoresis, or tremor. These do not usually bring the individual to medical attention or require treatment. Higher doses or repeated use is associated with more serious medical events, including nausea and vomiting, acute coronary syndrome (unstable angina or myocardial infarction, usually resulting in chest pain), cardiac tachyarrhythmia, hypertension, seizures, stroke, hyperthermia, or rhabdomyolysis (3,4,28,36–38). Acute medical complications associated with acute stimulant intoxication are summarized in Table 46-1.
Stimulant use should be high on the list of possible diagnoses for any younger patient presenting with one of these events, especially in the absence of other risk factors (39,40). Urine or blood samples for toxicologic analysis should be obtained to determine what drugs, if any, the patient has ingested recently. Even if an apparently adequate history has been obtained, the patient or collateral informants may not know the true content of any street drugs that have been used. A history of stimulant use within the preceding 96 hours or a positive toxicology test is highly suggestive. The actual blood cocaine concentration has little prognostic significance (41).
Nontraumatic chest pain is a common presenting complaint among stimulant users who seek acute medical care. The differential diagnosis includes acute coronary syndrome, acute aortic dissection, pneumothorax or pneumo-mediastinum (especially among drug smokers), endocarditis or pneumonia (especially among injection drug users), pulmonary embolus, myocarditis or cardiomyopathy, or musculoskeletal pain after a seizure (28,38). About 1% to 6% of patients with cocaine-associated chest pain and up to one-fourth of those with methamphetamine-associated chest pain will have an acute myocardial infarction (28,42). The risk for infarction is greatest during the first 1 to 3 hours after cocaine use and then declines rapidly (42). Concurrent use of multiple stimulants (e.g., cocaine and methamphetamine) may enhance cardiotoxicity (18), whereas concurrent use of opioids (such as “speedballing”) may mask the diagnosis (43).
The electrocardiogram is not always helpful diagnostically because of its low sensitivity and positive predictive value and the high frequency (more than one-third in some studies) of benign early repolarization among patients presenting with cocaine-associated chest pain (44,45). The best laboratory test for acute myocardial infarction is serial blood levels of cardiac troponin I (39,42). Its high specificity (around 95%) for acute myocardial infarction is not affected by recent cocaine use because it does not cross-react with skeletal muscle troponin. In contrast, myoglobin and creatine kinase (CK) levels may be elevated due to cocaine-associated rhabdomyolysis.
Patients who present with nontraumatic stimulant-associated chest pain usually should be observed for 9 to 12 hours while undergoing evaluation (46). Delayed complications are rare, so resolution of symptoms with a negative evaluation warrants discharge. Patients who have persistent chest pain despite standard treatment, hypotension, congestive heart failure, or cardiac arrhythmia require hospitalization for further evaluation and treatment. Even patients with confirmed acute myocardial infarction have a favorable prognosis, possibly because of their relatively young age and good underlying health (42).
Rhabdomyolysis may be due to a direct effect of the drug, hyperthermia, excessive muscle activity, or trauma (26). The usual symptoms of myalgia and muscle tenderness and swelling often are absent in rhabdomyolysis associated with stimulants. The diagnosis is suggested by a plasma CK level greater than five times normal (with other tissue sources ruled out) and a urine dipstick positive for heme but without red blood cells (indicating free myoglobin [or hemoglobin] in the urine).
Management of Medical Effects of Stimulant Intoxication
The first priority in the management of severe acute stimulant intoxication is maintenance of basic life support functions (29). Vital signs, hydration status, and neurologic status should be monitored closely. Activated charcoal or gastric lavage with isotonic saline may be helpful if a large amount of stimulant has been taken orally within the preceding hour (5,25). This can be done by oral intake or via a nasogastric tube in the awake, cooperative patient. Activated charcoal (50 to 100 g orally) may be just as effective as gastric lavage and minimizes the risk of aspiration. Ipecac-induced vomiting is not recommended.
Severe hypertension (e.g., diastolic blood pressure >120) that lasts more than 15 minutes should be treated promptly to avoid CNS hemorrhage (5). Rhabdomyolysis should be treated vigorously with intravenous fluid to maintain a urine output of greater than 2 mL/kg/h to avoid myoglobinuric renal failure (5,25). Maintenance of urine pH >5.6 with sodium bicarbonate (1 mmol/kg IV) helps to prevent the dissociation and precipitation of myoglobin.
Benzodiazepines in sedative doses are the initial treatment of choice for both acute cardiovascular and CNS toxicity from stimulants (39,44). Hypertension or tachycardia that does not respond to sedation alone may be treated with an alpha-adrenergic blocker such as phentolamine (2 to 10 mg IV over 10 minutes). Beta-adrenergic blockers such as propranolol or esmolol should be used with caution because of the risk of unopposed alpha-adrenergic stimulation by the stimulant, resulting in vasoconstriction and worsening hypertension (39,42,45,47). However, despite concerns about the use of beta blockers, a recent study has not found them to be associated with worse outcomes (48). The combined alpha-and beta-adrenergic blocker labetalol shows little alpha-adrenergic antagonism in clinical practice and also should be avoided. If alpha-adrenergic blockade is ineffective, direct vasodilation with sodium nitroprusside infusion (0.25 to 10 μg/kg/min) or nitroglycerin (5 to 100 μg IV) can be used. There is no evidence that rapid lowering of blood pressure compromises peripheral (including cerebral) circulation in an otherwise intact patient. Calcium channel blockers may reduce vasospasm, but their role remains unclear; calcium channel blockers enhance CNS toxicity in animal studies, have inconsistent effects in case series, and should be avoided in patients with heart block or heart failure. Dexmedetomidine, an α2-adrenergic receptor agonist, has shown promise in ameliorating the acute cardiovascular effects of severe cocaine intoxication, as well as providing sedation (49,50). It must be given by IV infusion (not bolus) to avoid hypertension and bradycardia.
Treatment of cocaine-induced cardiac tachyarrhythmias begins with correction of any exacerbating conditions such as myocardial ischemia, hypoxia, electrolyte abnormalities, or acid–base disturbance (42,46). Arrhythmias occurring several hours after cocaine use are usually secondary to myocardial ischemia. Standard arrhythmia management is usually appropriate, including use of lidocaine. Arrhythmias occurring immediately after cocaine use are usually from the sodium channel blocking action of cocaine. These may respond to sodium bicarbonate. Lidocaine (which also blocks sodium channels) should be used cautiously in this context because of animal studies suggesting it exacerbates cocaine-associated arrhythmias and seizures. Class IA antiarrhythmic medications (such as quinidine, procainamide, or disopyramide) should be avoided because of their potential additive effect on QRS and QT interval prolongation. There are no data on the use of amiodarone for cocaine-associated arrhythmias. Treatment of cardiac arrest is the same as for non–cocaine users; the outcome may be more favorable than for drug-free patients (51).
The treatment of stimulant-associated acute coronary syndrome largely resembles that for the non–drug-associated syndrome, with the exception of avoiding use of beta-adrenergic blockers and labetalol (46,47). Initial treatment includes oxygen, benzodiazepine for sedation, morphine for pain, sublingual nitroglycerin for vasodilation, and aspirin for antiplatelet action, while evaluation is continuing. Further treatment can include phentolamine or intravenous nitrates (10 μg/kg/min) to lower blood pressure and reverse coronary artery vasoconstriction. The role of calcium channel blockers is not well defined (39,42). They may be useful in patients who have not responded to benzodiazepines and nitroglycerin.
Both fibrinolytic therapy and percutaneous transluminal coronary angioplasty have a role in the treatment of confirmed myocardial infarction (39,42). Angioplasty may be preferable to fibrinolysis because of the increased risk of intracranial hemorrhage in cocaine users. Elevated body temperature (>102°F orally) is a marker for poor prognosis and should be managed aggressively to avert hyperthermic crisis (as by cold water sponging, cooling blankets, ice packs, ice water gastric lavage, or cold peritoneal lavage) (5,52). Untreated hyperthermia may result in rhabdomyolysis and renal failure.
Intravenous benzodiazepines (diazepam 5 to 10 mg or lorazepam 2 to 10 mg over 2 minutes, repeated as needed) are recommended to control seizures stemming from stimulant intoxication (49,52). Fosphenytoin (15 to 20 mg/kg at 100 to 150 mg/min) or phenobarbital (15 to 20 mg/kg over 20 minutes) also can be used. However, the latter may cause hypotension or prolonged sedation.
Excretion of amphetamine can be increased by acidifying the urine to pH <6.6 (as with 500 mg of oral ammonium chloride every 3 to 4 hours), which inhibits renal reabsorption of amphetamine (53). The actual clinical usefulness of this maneuver is uncertain (13). Acidification is contraindicated in the presence of myoglobinuria, if renal or hepatic function is abnormal, or in overdose situations, when plasma acidification may compromise cardiovascular function (33).
Stimulant Withdrawal
Abrupt cessation of stimulant use is associated with depression, anxiety, fatigue, difficulty concentrating, anergia, anhedonia, increased drug craving, increased appetite, hyper-somnolence, and increased dreaming (because of increased REM sleep) (54–56). The initial period of intense symptoms is commonly termed the “crash,” but most symptoms are mild and self-limited, resolving within 1 to 2 weeks without treatment.
Hospitalization for stimulant withdrawal is rarely indicated on medical grounds and has not been shown to improve the short-term outcome for stimulant addiction (57,58). Pharmacologic treatment has focused more on long-term treatment of addiction than on short-term treatment of acute withdrawal (59,60). Most clinical trials that used medication during the early withdrawal period have continued to use such medication for at least several weeks, with the additional goal of treating the addiction itself.
Medical Effects of Stimulant Withdrawal
The first week of stimulant withdrawal has been associated with myocardial ischemia (61), possibly because of coronary vasospasm. Other medical effects of stimulant withdrawal are relatively minor, including nonspecific musculoskeletal pain, tremors, chills, and involuntary motor movement (62). These rarely require specific medical treatment.
Management of Stimulant Withdrawal
The stimulant withdrawal syndrome has been hypothesized to be the result of decreased levels of brain dopamine activity resulting from chronic stimulant exposure. This so-called “dopamine deficiency” hypothesis of withdrawal has not been consistently supported by clinical studies (63–65), but has generated the use of dopamine agonists to treat cocaine withdrawal, most commonly bromocriptine and amantadine. However, no medication has been shown consistently effective in controlled clinical trials (66) or is any medication approved for the treatment of stimulant withdrawal by any national regulatory authority. Administration of a cross-tolerant or similarly acting stimulant has not been systematically evaluated as a short-term treatment for stimulant withdrawal (24). No controlled clinical trial has directly compared the benefits of medication versus a supportive milieu.
Symptoms of stimulant withdrawal are best treated supportively with rest, exercise, and a healthy diet (5,24). Short-acting benzodiazepines such as lorazepam may be helpful in selected patients who develop agitation or sleep disturbance. Severe (suicidal ideation) or persistent (>2 to 3 weeks) depression may require antidepressant treatment (5,66a) and psychiatric admission. The risk of relapse is high during the early withdrawal period, in part because drug craving is easily triggered by encounters with drug-associated stimuli. This issue is better addressed by psychosocial treatment, such as supportive therapy, cognitive–behavioral therapy, relapse prevention, and contingency management, than by medication.
HALLUCINOGENS
Hallucinogen Intoxication
Hallucinogens have in common the ability to change or distort sensory perceptions in a clear sensorium. Most hallucinogens fall into one of two chemical groups (see Chapter 14). Indolealkylamine hallucinogens (including LSD, psilocybin, or N,N-dimethyltryptamine) are structurally related to serotonin; phenylethylamine hallucinogens (including 3,4,5-tri-methoxyphenylethylamine [mescaline], 3,5- dimethoxy-4-methylamphetamine [DOM, STP]) are structurally related to norepinephrine. Both indolealkylamine and phenylethyl-amine hallucinogens generate psychedelic (LSD-like) experiences and thus are often categorized together (67,68). In contrast, 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) has characteristics of both a hallucinogen and a stimulant and is considered separately (see also Chapter 14). PCP and its close analog ketamine are anesthetics that are used for their dissociative and euphoric effects. Both MDMA and PCP are considered in their own section below (see also Chapter 15).
Psychological and Behavioral Effects of Hallucinogen Intoxication
The acute psychological and behavioral effects of hallucinogen intoxication are summarized in Table 46-3. The subjective experience is influenced greatly by set and setting, that is, the expectations and personality of the user, coupled with the environmental and social conditions of use. Mood can vary from euphoria and feelings of spiritual insight to depression, anxiety, and terror. Perception usually is intensified and distorted, with alterations in the sense of time, space, and body boundaries. While illusions (visual and auditory distortions of perception) are common, true hallucinations (perceptions that do not have any basis in reality) are not. Synesthesia, a blending of the senses wherein colors are heard and sounds are seen, is a common perceptual distortion. Cognitive function may range from clarity to confusion and disorientation, although reality testing usually remains intact.
TABLE 46-3 ACUTE PSYCHOLOGICAL AND BEHAVIORAL EFFECTS OF INTOXICATION WITH LSD, MARIJUANA, PCP, OR MDMA
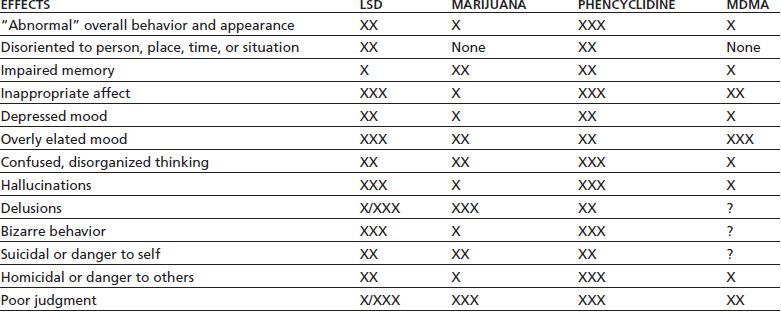
Relative weighting: X = mild; XX = moderate; XXX = marked; /= common/rare; ? = insufficient research.
MDMA, 3,4-methylenedioxymethamphetamine.
Sources: Brust JCM. Acute neurologic complications of drug and alcohol abuse. Neurol Clin N Am 1998;16:503–519; Frecska E, Luna LE. The adverse effects of hallucinogens from intramural perspective. Neuropsychopharmacol Hung 2006;8:189–200; Abraham HD, Aldridge AM, Gogia P. The psychopharmacology of hallucinogens. Neuropsychopharmacology 1996;14:285–298. Refs. (69–71).
A “bad trip” (69) usually takes the form of an anxiety attack or panic reaction, with the user feeling out of control (70,72,73). An experience of depersonalization may precipitate the fear of losing one’s mind permanently. Panic reactions are more common in those who have limited experience with hallucinogens, but previous “positive” experiences provide no protection against an adverse reaction (74). While higher doses are associated with more intense experiences, adverse reactions are less a function of dose than of context and environment. Hallucinogens may trigger a transient psychosis even in psychologically normal users; however, a true psychotic episode is rare. Hallucinogen-induced psychosis may resemble acute paranoid schizophrenia (68,74). The two usually can be distinguished because patients with schizophrenia tend to have auditory (rather than visual) hallucinations and a history of prior mental illness. Hallucinogen users, unlike patients with schizophrenia, usually retain at least partial insight that their symptoms are drug related.
Hallucinogen ingestion may result in an acute toxic delirium that is characterized by delusions, hallucinations, agitation, confusion, paranoia, and inadvertent suicide attempts (e.g., attempts to fly or perform other impossible activities).
Medical Effects of Hallucinogen Intoxication
Acute medical complications of hallucinogen intoxication are summarized in Table 46-4. Sympathomimetic effects are common, particularly pupillary dilation, hyperreflexia, piloerection, tachycardia, and increases in blood pressure. Dizziness, paresthesias, headache, nausea, or tremor may occur. Body temperature should be monitored and any elevation treated promptly. Dry skin, increased muscle tone, agitation, and seizures are warning signs of a potential hyperthermic crisis. Patients may not respond to anticonvulsant medication until body temperature is lowered. Complications that require treatment are rare in the absence of overdose (68,69).
Table 46-4 A cute Medical Complications of Intoxication with LSD, MDMA, Marijuana, or PCP
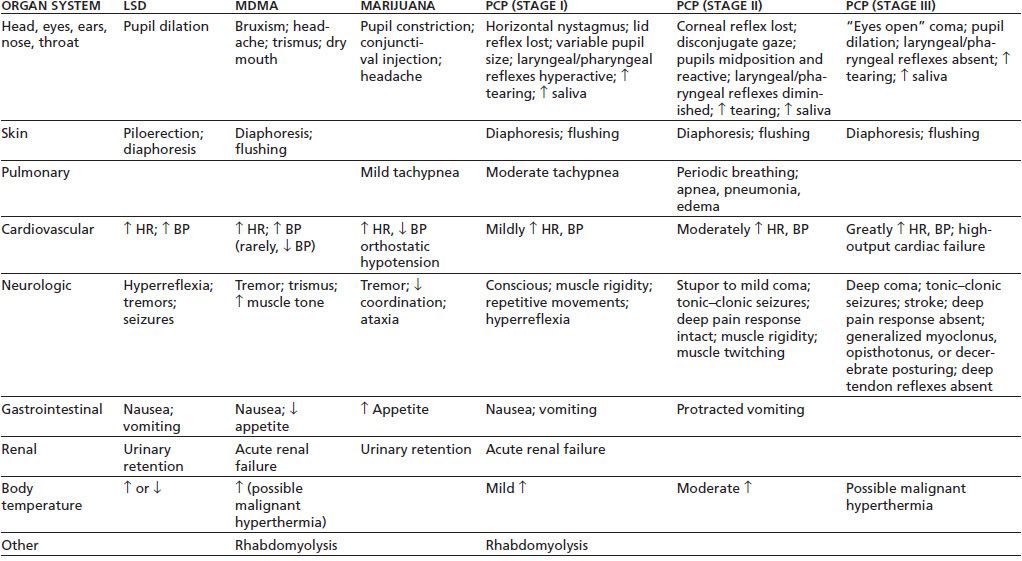
MDMA, 3,4-methylenedioxymethamphetamine; HR, heart rate; BP, blood pressure.
Sources: Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart 2000;83:627–633; Brust JCM. Acute neurologic complications of drug and alcohol abuse. Neurol Clin N Am 1998;16:503–519; Frecska E, Luna LE. The adverse effects of hallucinogens from intramural perspective. Neuropsychopharmacol Hung 2006;8:189–200; Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA)and related drugs. Can Med Assoc J 2001;165(7):917–928; Schuckit MA. Drug and alcohol abuse. A clinical guide to diagnosis and treatment, 6th ed. New York: Springer, 2006; Selden BS, Clark RF, Curry SC. Marijuana. Emerg Med Clin North Am 1990;8:527–539. Refs. (75,76).
Oral LSD is rapidly absorbed, so that ipecac-induced vomiting or gastric lavage usually is not helpful and may exacerbate the patient’s psychological distress. There is no evidence that LSD binds to charcoal. Gastric lavage may be useful in psilocybin ingestion or when there is doubt as to the identity of the ingested mushrooms (70,72).
Management of Hallucinogen Intoxication
Initial treatment is supportive. The patient should be placed in a quiet environment with minimal sensory stimulation, but should be observed because of the risk of unintended self-injury (as the result of delusions or hallucinations) or of suicide (as the result of depression). The presence of a familiar person usually is comforting. Unless the patient presents in an acutely agitated or threatening state, physical restraints are contraindicated because they may exacerbate anxiety and increase the risk of rhabdomyolysis associated with muscle rigidity or spasms. The use of “gentle restraints” in combination with muscle massage and individualized counseling may be helpful (74).
The “talk down” or reassurance technique may be helpful. The clinician, in a concerned and nonjudgmental manner, discusses the patient’s anxiety reaction, stressing that the drug’s effects are temporary and that the patient will recover completely.
For patients who do not respond to reassurance alone, oral benzodiazepines such as lorazepam (1 to 2 mg) or diazepam (10 to 30 mg) are the drugs of choice (68,72). When oral medication is too slow, or the patient will not take oral medication, intramuscular lorazepam (2 mg, repeated hourly as needed) may be effective. If benzodiazepines are insufficient, a high-potency antipsychotic such as haloperidol (5 to 10 mg orally or 2 mg intramuscularly) may be needed. The role of second-generation antipsychotics in this situation remains unclear, but 5-HT2A receptor antagonism may be a useful property (68,72,73). Phenothiazines should be avoided because they are associated with poor outcomes (77) and may exacerbate unsuspected anticholinergic poisoning.
Patients usually recover sufficiently after several hours and may be released into the care of a responsible relative or friend. If psychosis does not resolve within 1 or 2 days, ingestion of a longer-acting drug such as PCP or DOM should be suspected (5). Symptoms that persist beyond a few days raise the strong likelihood of a preexisting or concurrent psychiatric or neurologic condition. Psychiatric problems that last more than a month probably are related to preexisting psychopathology.
Treatment for hallucinogen-induced delirium generally follows the guidelines for simple intoxication: Isolate the patient, and minimize sensory input until effects of the drug have worn off. Reassurance that the delirium will abate as the drug is metabolized also may be helpful. Pharmacologic treatment is not necessary in most cases and may confuse the clinical picture. If medication is needed, a drug with few anticholinergic properties is preferred for the reasons listed above; for example, diazepam may be given 15 to 30 mg orally, repeating 5 to 20 mg every 3 to 4 hours as needed.
Hallucinogen Withdrawal
Withdrawal symptoms, including fatigue, irritability, and anhedonia, are reported by about 10% of hallucinogen users (78). There is no evidence to suggest a clinically significant hallucinogen withdrawal syndrome (62,68), and such a syndrome is not recognized in the DSM-5 (79). The rapid development of tolerance (within 3 to 4 days) may explain in part why use of LSD-like drugs generally is intermittent. There is no role for medication in the treatment of hallucinogen withdrawal.
Some users of hallucinogens describe experiencing flashbacks, vivid memories, or brief recurrences of sensory distortions reminiscent of intoxication, during periods of sobriety. Flashbacks can occur spontaneously long after cessation of use and thus are not truly a withdrawal syndrome. In the DSM-5, they are diagnosed as “hallucinogen persisting perception disorder” (79). Initiation of selective serotonin reuptake inhibitors (SSRIs) or neuroleptics is associated with recurrences of flashbacks in at-risk individuals (80,81). Supportive measures, as well as the symptom-based pharmacologic interventions prescribed to manage hallucinogen intoxication, may be effective in managing such symptoms.
MARIJUANA
Marijuana Intoxication
The major psychological and physiologic effects of marijuana are mediated by the interaction of delta-9-tetrahydrocannabinol (THC) with specific cannabinoid (CB1) receptors on nerve cells (82,83), the regional distribution of which in the human brain is consistent with the known effects of marijuana (84). Other cannabinoids found in marijuana (e.g., cannabidiol, cannabinol) do not produce these typical marijuana effects (85). In animal and human studies, acute THC effects are reduced or blocked by CB1 receptor antagonists (86).
Psychological and Behavioral Effects of Marijuana Intoxication
The initial—usually desired—psychological effects of marijuana intoxication include relaxation, euphoria, slowed time perception, altered (often intensified) sensory perception, increased awareness of the environment, and increased appetite (87). Undesired effects may include impaired concentration, anterograde amnesia (88), and motor incoordination (89). As with hallucinogens, psychological set and social setting and prior experience with the drug can substantially influence the quality of the experience. Higher doses, repeated use, or a stressful setting is associated with adverse effects such as hypervigilance, anxiety, paranoia, derealization and depersonalization (commonly associated with altered time sense), acute panic (associated with anxiety), illusions or hallucinations (usually auditory or visual), psychosis, or delirium (68,90–92,92a). Acute marijuana- associated psychosis can be difficult to distinguish from schizophrenic psychosis other than by its transient time course (93). Marijuana-associated psychosis may be more likely to exhibit derealization/depersonalization experiences and visual, rather than auditory, hallucinations. Preexisting psychopathology increases the risk of adverse events such as panic attack or psychosis (94). Table 46-3 summarizes the acute adverse psychological effects of marijuana intoxication. Oral ingestion of marijuana can produce the same adverse reactions as does smoking, including psychosis (92a,95,96).
Medical Effects of Marijuana Intoxication
The acute physiologic effects of oral or smoked marijuana intoxication include conjunctival injection (“red eye”) due to vasodilation, tachycardia (sometimes with palpitations), orthostatic hypotension (sometimes resulting in syncope), and dry mouth (see Table 46-4). Neurologic signs include poor motor coordination, head jerks, and impairment of smooth pursuit eye movements (89). These generally are mild, are self-limiting, and do not require medical treatment (87). There are no well-established cases of human fatalities from exclusively marijuana overdose (91), although several cases of possible acute cardiovascular death have been reported (97) and marijuana smoking has been associated with atrial fibrillation and other tachyarrhythmias (98,99). Intravenous use of marijuana, although rare, can be associated with cardiovascular shock and renal failure (99).
Management of Marijuana Intoxication
Adverse effects of marijuana intoxication tend to be self-limited and often can be managed without medication. The patient should be kept in a quiet environment and offered supportive reassurance. If immediate pharmacologic intervention is needed to control severe agitation or anxiety, benzodiazepines are preferred to antipsychotics, although there are no controlled studies to confirm this. Psychosis usually responds to low doses of second-generation antipsychotics (92).
No medication is approved by any national regulatory authority for the treatment of marijuana intoxication. The selective cannabinoid CB1 receptor antagonist/inverse agonist rimonabant (developed for weight loss) blocked the acute psychological and cardiovascular effects of smoked marijuana in human laboratory studies (87). However, rimonabant and several similar medications were withdrawn from the market and from clinical development in 2008 because of psychiatric side effects (100). Should a future CB1 receptor antagonist prove safe and effective, it could be used to treat acute marijuana intoxication in the same way that naloxone acts on opiate intoxication.
Marijuana Withdrawal
Acute marijuana withdrawal is reported by up to one-third of heavy marijuana users in the community and more than half of those seeking treatment for marijuana dependence (101) and is a recognized clinical syndrome in the latest (fifth) edition of the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (79). Symptoms are primarily psychological, including irritability, anxiety, depression, restlessness, anorexia, insomnia, and disturbed sleep (102). Much less common are physical symptoms such as gastrointestinal distress, diaphoresis, chills, nausea, shakiness, muscle twitches, and increased blood pressure (102). The syndrome is usually mild, comparable to tobacco withdrawal (103), and rarely needs medical attention, but may impair some normal activities of daily life (104). It may warrant clinical attention in the treatment of cannabis use disorders because withdrawal symptoms can serve as negative reinforcement for relapse among users trying to maintain abstinence (105).
Management of Marijuana Withdrawal
Marijuana withdrawal rarely requires treatment for intrinsic medical or psychiatric reasons, although treatment might be warranted in some cases to reduce the risk of relapse in persons trying to abstain while experiencing distressing withdrawal symptoms. In two controlled clinical trials involved treatment-seeking adults with cannabis dependence, both dronabinol (synthetic THC, 20 mg b.i.d.) and gabapentin (1,200 mg daily) significantly reduced marijuana withdrawal symptoms, although only gabapentin significantly reduced marijuana use (106).
DISSOCIATIVE ANESTHETICS
Phencyclidine, Ketamine, and Dextromethorphan Intoxication
PCP and its molecular analog ketamine are dissociative anesthetics (107,108); DXM is widely available as an antitussive in over-the-counter cough and cold medicines (109). The chemical agents in this class are relatively old, with PCP first synthesized just under 90 years ago (110) and both ketamine (111) and DXM approximately 50 years ago (112). Of the three, ketamine has received considerable attention in recent years because of its apparent ability to rapidly treat unipolar (113) and bipolar (114) depression and various pain syndromes (115). There is a rich literature for the abuse of these three dissociative agents. Both PCP and ketamine are considered controlled drugs in the United States; PCP is a Schedule II and ketamine is a Schedule III drug (116). The related drug DXM is not controlled and is widely available as an ingredient in over 100 different over-the-counter cough and cold medicines (117). At the recommended antitussive dose of 15 to 30 mg every 6 to 8 hours, adverse reactions are rare. However, about 5% to 10% of those of white European ethnicity are unable to demethylate DXM to dextrorphan (an active metabolite) because of a deficit in the liver cytochrome P450 CYP 2D6 isoenzyme. Thus, in the context of megadose use of DXM, this subset of individuals is at increased risk of toxicity from an acute excess in DXM levels (117).
The main effects of PCP and ketamine are mediated by their action as noncompetitive antagonists of the NMDA glutamate excitatory amino acid neurotransmitter receptor (111). In addition, direct effects on other neurotransmitter systems (such as dopamine) may occur at high doses (111,118) (see Chapter 15). In addition to NMDA antagonism, DXM has activity at the sigma receptor, which likely contributes to its therapeutic effects as a cough suppressant.
Psychological and Behavioral Effects of Dissociative Anesthetic Intoxication
Dissociative anesthetics produce a range of intoxicated states that can be grouped into three stages (118): Stage I, conscious, with psychological effects but (at most) mild physiologic effects; Stage II, stuporous or in a light coma, yet responsive to pain; and Stage III, comatose and unresponsive to pain. Table 46-3 summarizes the acute psychological and behavioral effects of PCP intoxication and overdose. The time course of psychological effects is highly variable and unpredictable, so that even a recovering patient should be kept under observation until all symptoms have resolved (typically at least 12 hours) (119). Patients may “emerge” from one stage of intoxication to the next; that is, a stuporous or comatose patient in Stage II or III may enter Stage I and become agitated and delirious (68,120). Similarly, a conscious patient in Stage I may suddenly become comatose (5). The entire clinical episode may require up to 6 weeks to resolve (5).
The psychiatric manifestations of Stage I intoxication can resemble a variety of psychiatric syndromes, making differential diagnosis difficult in the absence of toxicology results or a history of recent PCP, ketamine, or DXM intake. Common syndromes seen in treatment settings include delirium, psychosis without delirium, catatonia, hypomania with euphoria, and depression with lethargy. Agitated or bizarre behavior, with increased risk of violence, can occur with any psychiatric presentation (68,77,95,112). Because of the analgesic effect of PCP, patients may not report the existence of even serious injuries (which may be self-inflicted) (69).
Clinically significant psychological and behavioral effects of DXM begin to occur at approximately five times the therapeutic dose (117). These effects can be grouped into four dose-dependent plateaus (Table 46-5).
TABLE 46-5 PSYCHOLOGICAL AND BEHAVIORAL EFFECTS OF DXM INTOXICATION
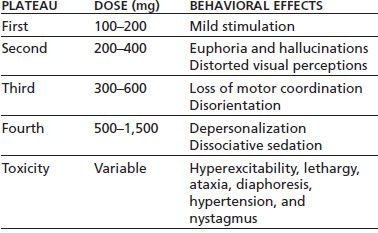
Adapted from Schwartz RH. Adolescent abuse of dextromethorphan. Clin Pediatr 2005;44(7):565–568.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


