Cellular Homeostasis
Mg is compartmentalized within the cell, and most of it is bound to proteins and negatively charged molecules. Significant amounts of Mg are found in the nucleus, mitochondria, the endoplasmic and sarcoplasmic reticulum, and the cytoplasm (
5,
6,
20). Total cell Mg concentration has been reported to range between 5 and 20 mM (
15). From 90% to 95% of cytosolic Mg is bound to ligands such as ATP, adenosine diphosphate (ADP), citrate, proteins, and nucleic acids. The remainder is free Mg
2+, constituting 1% to 5% of the total cellular Mg (
15,
21).
The concentration of free ionized Mg
2+ in the cytoplasm of mammalian cells has ranged from 0.5 to 1.0 mM, similar to circulating ionized Mg
2+ (
6,
15). The Mg
2+ concentration in the cell cytoplasm is maintained relatively constant even when the Mg
2+ concentration in the extracellular fluid is experimentally varied to either high or low nonphysiologic concentrations (
22). The relative constancy of the Mg
2+ in the intracellular milieu is attributed to the limited permeability of the plasma membrane to Mg and to the operation of specific Mg transport proteins, which regulate the rates at which Mg is taken up or extruded from cells (
5,
6,
15). Maintenance of a normal intracellular concentration of Mg
2+ requires that Mg be actively transported out of the cell (
15). Mg transport into or out of cells appears to require the presence of carriermediated transport systems. The efflux of Mg from the cell appears to be coupled to Na transport and requires extrusion of Na by Na
+/K
+-ATPase (
15). Evidence also indicates a Na-independent efflux of Mg (
7,
15). Mg influx appears to be linked to Na transport, but by a different mechanism than efflux (
15,
23). At least seven transmembrane Mg
2+ channels have been cloned (
24). These include NIPA2 (
25) and MagT1 and TUSC3 (
26). Studies of human hereditary diseases (see later) have identified paracellin-1 (claudin 16), claudin 19, and two transient receptor potential channel family members, TRPM6 and TRPM7 (
27,
28,
29). TRPM6 is expressed in the kidney, and TRPM7 is constitutively expressed (
28). Tissues vary with respect to the rates at which Mg exchange occurs and the percentage of total Mg, which is readily exchangeable (
7). The rate of Mg exchange in heart, liver, and kidney exceeds that in skeletal muscle, lymphocytes, red blood cells, brain, and testis.
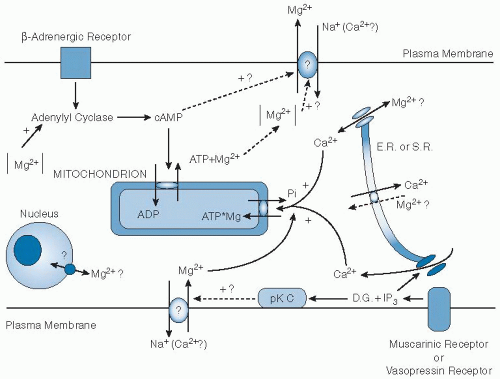
The processes that maintain or modify the relationships between total and ionized internal and external Mg are incompletely understood. Changes in cytosolic Mg
2+ regulate some channels (TRPM6 and TRPM7) (
24). Mg transport in mammalian cells may be influenced by hormonal and pharmacologic factors (
15). Mg
2+ efflux was stimulated after short-term exposure of isolated perfused rat heart and liver or thymocytes to α and β-agonists and permeant cAMP (
30,
31). Activation of protein kinase C by diacylglycerol or by phorbol esters stimulates Mg
2+ influx and does not alter efflux (
32). Epidermal growth factor (EGF) has been shown to increase Mg
2+ transport into a vascular smooth muscle cell line (
33). Insulin and dextrose were found to increase
28Mg uptake by several tissues, including skeletal and cardiac muscle (
5,
6). The mechanism of insulin-induced Mg transport is likely the result of an effect on protein kinase C (
5,
6). An insulininduced transport of Mg into cells could be one factor responsible for the fall in the serum Mg concentration observed during insulin therapy of diabetic ketoacidosis (
34). Investigators have hypothesized that this hormonally regulated Mg uptake system controls intracellular Mg
2+ concentration in cellular subcytoplasmic compartments. The Mg
2+ concentration in these compartments would then serve to regulate the activity of Mg-sensitive enzymes. An overall schema of cellular Mg homeostasis is shown in
Figure 9.1.