CHAPTER 5 Macromolecular Assembly
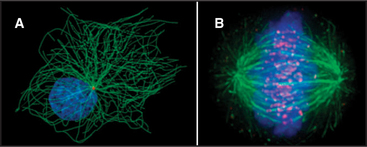
The discovery that dissociated parts of viruses can reassemble in a test tube led to the concept of self-assembly, one of the central principles in biology. In vitro analysis of true self-assembly from purified components of viruses, bacterial flagella, ribosomes, and cytoskeletal filaments has revealed the general properties of these processes. For example, large biological structures, such as the mitotic spindle (Fig. 5-1), are constructed from molecules that assemble by defined pathways without the aid of templates. Even large cellular components, such as chromosomes, nuclear pores, transcription initiation complexes, vesicle fusion machinery, and intercellular junctions, assemble by the same strategy. The properties of the constituents determine the assembly mechanism and architecture of the final structure. Weak but highly specific noncovalent interactions hold together the building blocks, which include proteins, nucleic acids, and lipids.
Assembly of macromolecular structures differs fundamentally from the template-specified, enzymatic mechanisms with which cells replicate genes (see Chapter 42) and translate genes into RNAs and proteins (see Chapters 15 and 17). Macromolecular assembly does not require templates and rarely involves enzymatic formation or dissolution of covalent bonds. When enzymatic processing occurs during the assembly of some viruses (see Example 7 later in the chapter, in the section titled “Regulation by Accessory Proteins”), collagen (see Fig. 29-6), and elastin (see Fig. 29-11), it usually precludes reassembly of the dissociated parts.
Assembly of Macromolecular Structures from Subunits
The use of subunits provides multiple advantages for assembly processes, as was originally pointed out by Crane (Box 5-1). These advantages include the following:
BOX 5-1 Crane’s Hypothesis
In 1950, the physicist H. R. Crane predicted in Scientific Monthly that all macromolecular structures in biology are assembled from multiple subunits and according to the laws of symmetry. A symmetric structure is composed of numerous identical subunits, all in equivalent environments (i.e., making identical contacts with their neighbors). For example, Figure 5-2A shows a plane hexagonal array, with each subunit making identical contacts with the six surrounding subunits. This is the most efficient way to fill a flat surface with globular subunits.
Crane also predicted that elongated tubular structures are assembled with symmetry. This type of symmetry is known as a helix. One way of constructing a helix is to take a plane hexagonal array, cut it along one of its lattice lines, and roll it up into a tube (Fig. 5-2B). The bonds between adjacent subunits are nearly identical in the plane array and the helical tube, except for the fact that each bond is distorted just enough to roll the sheet into a tube. Introduction of fivefold vertices into a hexagonal array allows it to fold up into a closed polygon (Fig. 5-2D–F).
Assembly of large structures from subunits conserves the genome. The assembly of macromolecular structures from identical subunits, like bricks in a wall, obviates the need to specify separate parts. For example, a plant virus, the tobacco mosaic virus (TMV; see Example 4 in this chapter), consists of 2130 protein subunits of 158 amino acids and a single-stranded RNA molecule of 6390 nucleotides. Having a separate gene for each viral coat protein would require 1,009,620 nucleotides of RNA, which would be about 160-fold longer than the entire viral RNA! The virus conserves its genome by using a single copy of the coat protein gene (474 nucleotides—7.4% of the genome) to make 2130 identical copies of protein that assemble into the virus coat.
Using small subunits improves the chance of synthesizing error-free building blocks. All biological processes are susceptible to error, and protein synthesis by ribosomes is no exception (see Chapter 17). The error rate of translation is about 1 in 3000 amino acid residues. Therefore, the odds that any given amino acid residue is correct are 0.99967. With these odds, the chance that a TMV subunit will be translated correctly is 0.99967158, or 0.949. Thus, about 95% of all TMV coat proteins in an infected cell are perfect, providing an ample supply of subunits with which to construct an infectious virus. Of the 5% of subunits with a mistake, some will be functional and others will not, depending on the nature and position of the amino acid substitution. Some amino acid substitutions pass unnoticed, whereas others result in loss of function. By contrast, the chance of correctly synthesizing the viral coat, if TMV coated its RNA with one huge polypeptide with 336,540 residues, would be only 0.99967336540, or 1.87 × 10−49.
Construction from subunits provides a mechanism for eliminating faulty components. Given that a significant fraction of all proteins have minor errors, good and bad subunits can be segregated on the basis of their ability to form correct bonds with their neighbors at the time of assembly. Many faulty subunits will not bond and thus are simply excluded from the final structure.
Subunits can be recycled. Many macromolecular structures assemble reversibly, and because they are built of subunits, the subunits can be reused later. For example, the subunits of the mitotic spindle microtubules reassemble into the interphase array of microtubules (Fig. 5-1; see also Chapter 44). Subunits in actin (see Example 1) and myosin (see Example 2) filaments are also recycled.
Assembly from subunits provides multiple opportunities for regulation. Simple modifications of subunits can regulate the state of assembly. For example, many intermediate filaments disassemble during mitosis when their subunits are phosphorylated by protein kinases (see Figs. 35-4 and 44-6).
Specificity by Multiple Weak Bonds on Complementary Surfaces
Stable macromolecular assemblies require intermolecular interactions stronger than the forces tending to dissociate the subunits. Subunits diffusing independently in an aqueous milieu have a kinetic energy of about 2.5 kJ mol−1 at 25°C. Interactions in macromolecular assemblies must be strong enough to overcome this thermal energy, which tends to pull them apart. Forces holding subunits together can be estimated from analysis of atomic structures (see Examples 1, 5, and 6) and the effects of solution conditions on the stability of assemblies (see Example 2).
Subunits of macromolecular assemblies are usually held together by the same four weak interactions (see Fig. 4-4) that stabilize folded proteins: the hydrophobic effect, hydrogen bonds, electrostatic interactions, and van der Waals interactions. Although none of these interactions is particularly strong on its own, stable association of macromolecular subunits is achieved by combining the effects of multiple weak interactions. This is possible because the free energy changes contributed by each weak interaction are added together. With a small correction for entropy changes, the overall binding constant for the association of subunits is the product of the equilibrium constants for each weak interaction [KA = (K1)(K2)(K3)(…)(Kn)].
Far from being a liability, multiple weak interactions provide assembly systems with the ability to achieve exquisite specificity that is derived from the “fit” between complementary surfaces of interacting molecules (see Examples 4 and 5). Complementary surfaces are important for three reasons. First, atoms that have the potential to form hydrogen bonds or electrostatic bonds must be placed in a complementary arrangement for the bonds to form. Second, complementary surfaces can exclude water between subunits, as required for the hydrophobic effect. Third and most important, repulsive forces arising from collisions between even a few atoms on imperfectly matching surfaces are strong enough to effectively cancel interactions between two potential bonding partners.
Many assembly reactions take advantage of flexibility in the protein subunits. In viral capsids (see Examples 5 and 6), hinges between the domains of the protein subunits provide the necessary flexibility to allow them to fit into more than one geometrical position. In some assemblies, flexible polypeptide strands knit subunits together (see Examples 1, 5, and 6). In other cases, assembly is coupled to the folding of the subunit proteins (see Examples 3, 4, and 6).
Symmetrical Structures Constructed from Identical Subunits with Equivalent (or Quasi-equivalent) Bonds
Studies of relatively simple systems composed of identical subunits, such as viruses and bacterial flagella, have provided most of what is known about assembly processes. The symmetry of these structures makes them ideal for analysis by X-ray crystallography and electron microscopy, and their biochemical simplicity facilitates analysis of assembly mechanisms. Subunits in asymmetric assemblies, such as transcription factor complexes (see Fig. 15-8), are likely to interact in the same way.
Subunits Arranged in Hexagonal Arrays in Plane Sheets
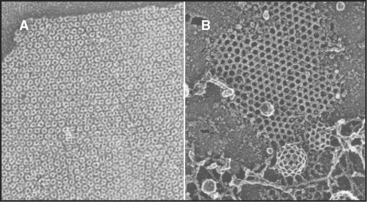
The simplest way to pack globular subunits in a plane is to form a hexagonal array with each subunit surrounded by six neighbors. This happens if one puts a layer of marbles in the bottom of a box and then tilts the box. A hexagonal array maximizes contacts between the surfaces of adjacent subunits. Membranes are the only flat surfaces in cells, and a number of membrane proteins crowd together in hexagonal arrays on or within the lipid bilayers. Connexons of gap junctions (Fig. 5-3), bacteriorhodopsin of purple membranes (see Fig. 7-7), and porin channels of bacterial membranes (see Fig. 7-7) all form regular hexagonal arrays in the plane of the lipid bilayer. Clathrin coats form hexagonal nets on the surface of membranes (Fig. 5-3).
Helical Filaments Produced by Polymerization of Identical Subunits with Like Bonds
Helical arrays of identical subunits form cytoskeletal filaments (see Examples 1 and 2), bacterial flagella (see Example 3), and some viruses (see Example 4). In helice subunits are positioned like steps of a spiral staircase. Each subunit is located a fixed distance along the axis and rotated by a fixed angle relative to the previous subunit. Helices can have one or more strands. TMV has one strand of subunits (see Example 4), whereas bacterial flagella have 11 strands (see Example 3). Helices can be either solid, like actin filaments (see Example 1), or hollow, like bacterial flagella (see Example 3) and TMV (see Example 4).
The asymmetry of protein subunits gives most helical polymers in biology a polarity (see Examples 1, 3, and 4). Different bonding properties at the two ends of the polymer have important consequences for their assembly and functions. Myosin filaments (see Example 2) have a bipolar helix, a rare form of symmetry. (The DNA double helix [see Fig. 3-3] is geometrically symmetric, with one strand running in each direction, but the order of its nucleotide subunits gives each strand a polarity.)
Spherical Assemblies Formed by Regular Polygons of Subunits
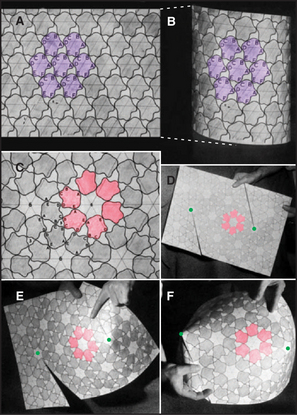
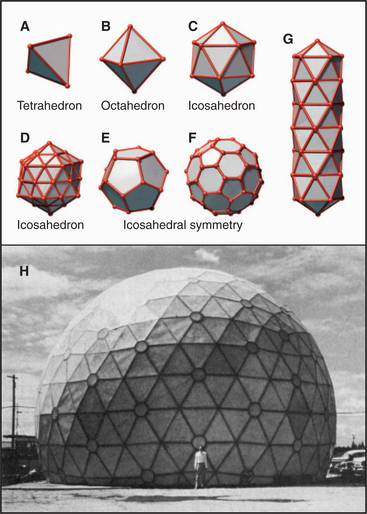
Geometric constraints limit the ways that identical subunits can be arranged on a closed spherical surface with equivalent or nearly equivalent contacts between the subunits. By far, the most favored arrangement is based on a net of equilateral triangles. On a plane surface, these triangles will pack hexagonally with sixfold vertices (Fig. 5-2). Since the time of Plato, it has been appreciated that introducing vertices surrounded by three, four, or five triangles will cause such a network of triangles to pucker and, given an appropriate number of puckers, to close up into a complete shell (Fig. 5-4). Four threefold vertices make a tetrahedron, six fourfold vertices make an octahedron, and 12 fivefold vertices make an icosahedron. Remarkably, no other ways of arranging triangles will complete a shell. In addition to threefold, fourfold, or fivefold vertices that introduce puckers, a closed polygon can contain additional triangular faces and sixfold vertices to expand the volume. The sixfold vertices can be placed symmetrically with respect to the fivefold vertices to produce a spherical shell or asymmetrically to form an elongated structure (Fig. 5-4G).
Most closed macromolecular assemblies in biology are polygons with fivefold vertices (see Examples 5 to 7). (The cubic iron-carrying protein ferritin is an exception.) An important reason for this is that most structures require some sixfold vertices to provide sufficient internal volume. This favors fivefold vertices for the puckers, as they require much less distortion of the subunits located on the triangular faces of the hexagonal plane sheet than do threefold or fourfold vertices. Further, the distortion in the contacts between the triangles is minimized if the fivefold vertices are in equivalent positions. Closed icosahedral shells can be assembled from any type of asymmetrical subunit given two provisions: (1) The subunit must be able to form bonds with like subunits in a triangular network; and (2) these subunits must be able to accommodate the distortion required to form both fivefold and sixfold vertices. Both fibrous (Fig. 5-5B) and globular subunits (see Examples 5 to 7) can fulfill these criteria.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree