Lymphomas Associated with FGFR1 Abnormalities
Roberto N. Miranda, MD
Key Facts
Terminology
Lymphoblastic lymphoma occurring in patients with 8p11 myeloproliferative syndrome
EMS is aggressive disease associated with FGFR1 gene abnormalities; diagnostic features include
Myeloproliferative neoplasm usually associated with dysplasia and eosinophilia
Lymphadenopathy usually due to T-LBL or bilineal T-cell/myeloid lymphoma
Frequent progression to acute myeloid leukemia
Synonym of 8p11 myeloproliferative syndrome
Myeloid and lymphoid neoplasms with FGFR1 abnormalities (WHO, 2008)
Clinical Issues
Lymphadenopathy is common
Cases associated with t(8;13)(p11;q12)
Leukocytosis is common at presentation
Poor prognosis despite aggressive chemotherapy
Stem cell transplantation may lead to long-term remission
Microscopic Pathology
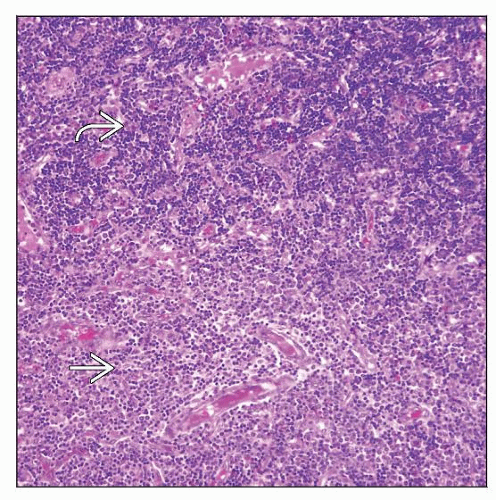
Diffuse or partial effacement of architecture by blasts
Mature eosinophils are commonly admixed within neoplasm
In some cases, biphasic pattern can be observed
Sheets of lymphoblasts
Larger cells, often perivascular, with moderate eosinophilic cytoplasm
Ancillary Tests
Chromosome 8p11/FGFR1 gene abnormalities
TERMINOLOGY
Abbreviations
Fibroblast growth factor receptor 1 (FGFR1) abnormalities
8p11 myeloproliferative syndrome (EMS)
Synonyms
Bilineal lymphoma or blastic T-cell/myeloid lymphoma
T-lymphoblastic leukemia/lymphoma ± eosinophilia
Synonyms of 8p11 myeloproliferative syndrome
Myeloid and lymphoid neoplasms with FGFR1 gene abnormalities
2008 World Health Organization classification
8p11 stem cell leukemia/lymphoma syndrome
Definitions
Lymphoblastic lymphoma occurring in patients with 8p11 myeloproliferative syndrome
T-cell, T-cell/myeloid, or rarely B-cell lineage reported in literature
Definition of 8p11 myeloproliferative syndrome
Clinically aggressive disease associated with FGFR1 gene abnormalities
Diagnostic features include
Myeloproliferative neoplasm usually associated with dysplasia and eosinophilia
Lymphadenopathy usually due to T-lymphoblastic leukemia/lymphoma
Frequent progression to acute myeloid leukemia
ETIOLOGY/PATHOGENESIS
Cell of Origin
Unknown but suspected to be pluripotent (lymphoid/myeloid) stem cell
CLINICAL ISSUES
Epidemiology
Age
Range: 3-84 years; median: 44 years
Gender
Slight male predominance
Presentation
Patients may present with fatigue, night sweats, weight loss, or fever
˜ 20% of patients are asymptomatic, and disease is discovered incidentally
Most patients present with lymphadenopathy
Usually generalized but can be localized
Hepatomegaly, splenomegaly, and hepatosplenomegaly are common
Extranodal sites of disease are uncommon
Sites reported: Tonsil, lung, and breast
Laboratory Tests
Leukocytosis is common at presentation; median: 46 x 109/L
Neutrophilia, eosinophilia, and monocytosis are common
Anemia or thrombocytopenia in ˜ 50% of patients
Natural History
Common evolution to acute leukemia of myeloid or mixed lineage
Treatment
Various protocols for acute leukemia have been used and have not been effective
Early stem cell transplantation may lead to long-term remission
Prognosis
Poor despite aggressive chemotherapy
Most patients die of disease
MICROSCOPIC PATHOLOGY
Histologic Features
Lymph node
Diffuse or partial effacement of architecture
Paracortical distribution in cases with partial involvement
Neoplastic cells are blasts that may show single file pattern of infiltration
Mature eosinophils are commonly admixed within neoplasm
Prominent high endothelial venules are common
In some cases, biphasic pattern with 2 components can be observed
Sheets of cells that are consistent with lymphoblasts (appear darker)
Larger cells with moderately abundant eosinophilic cytoplasm (appear pale)
Bone marrow
Usually hypercellular, eosinophilia is common
Blast count usually normal or slightly increased
˜ 15% of cases reported had > 20% blasts
Blasts are usually of myeloid or myeloid/lymphoid lineage
Features raise suspicion for myeloproliferative or myeloproliferative/myelodysplastic neoplasm
Peripheral blood smear
Leukocytosis with left shift in granulocyte maturation; ± blasts
Eosinophilia very common; ± monocytosis
ANCILLARY TESTS
Immunohistochemistry
Many cases of lymphoma in EMS reported as T-lymphoblastic leukemia/lymphoma
T-cell antigens(+), TdT(+), CD1a(+), Ig(-), B-cell antigens(-)
For cases of bilineal lymphoma in EMS that have 2 morphologic components
Myeloid cells express 1 or more myeloid-associated antigens
Myeloperoxidase(+/-), CD68(+/-), CD117(+/-), lysozyme(+/-), CD15(-/+)
Lymphoblasts: T-cell antigens(+), TdT(+), CD1a(+)
Flow Cytometry
Suspicion of EMS is helpful to ensure analysis of lymphoid and myeloid components
Blasts are usually positive for T-lineage markers, TdT, and CD1a
Cytogenetics
All cases of EMS carry abnormality involving FGFR1 gene at chromosome 8p11
10 translocations and 1 insertion have been identified
t(8;13)(p11;q12) is most common
Translocations are usually detected by conventional cytogenetic analysis; rarely are there cryptic translocations
Additional cytogenetic abnormalities are associated with progression to acute leukemia
Trisomy 21, in particular, is linked to progression
Molecular Genetics
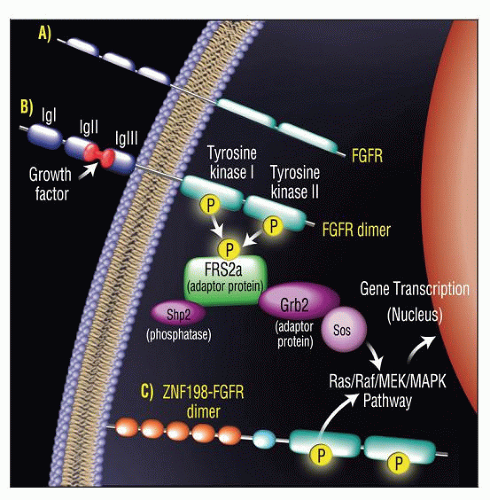
As consequence of 8p11 abnormalities, FGFR1 gene is disrupted
Results in creation of novel fusion genes and chimeric proteins
Chimeric proteins include portions of N-terminal partner gene and C-terminal portion of FGFR1
Partner genes and proteins foster dimerization and constitutional activation of FGFR1 tyrosine kinase domain
FISH and RT-PCR can be used to detect these translocations/gene rearrangements
Because of rarity of disease, these tests are not routinely available
Most cases of lymphoma carry monoclonal T-cell receptor (TCR) gene rearrangements
Subset of cases lack TCR gene rearrangements
Suggests that neoplastic transformation occurs at stem cell stage, before gene rearrangements occur
Patients with EMS have clinicopathological manifestations that correlate with specific molecular abnormalities
ZNF198-FGFR1: Lymphoma
FOP-FGFR1: Polycythemia, eosinophilia, older patient age
CEP110-FGFR1: Monocytosis, tonsillar involvement
BCR-FGFR1: Chronic myelogenous leukemia-like syndrome
DIFFERENTIAL DIAGNOSIS
Myeloid Sarcoma (MS)
Usually, underlying myeloproliferative neoplasm (MPN) or acute leukemia (AL)
Less frequently, myelodysplastic syndrome (MDS) or MDS/MPN
Association with characteristic cytogenetic or molecular abnormalities of underlying disease
Approximately 5% of AML cases can present as MS
MS can be nodal or extranodal
Nodal involvement is localized rather than generalized
Histologically there is diffuse infiltrate of intermediate to large myeloblasts or immature myelomonocytes
Immunophenotype: Lysozyme(+), CD68(+), myeloperoxidase(+), CD117(+)
Frequently CD13(+) and CD33(+)
CD34(+/-), CD99(+/-)
Cytochemistry on touch imprints is useful to define myeloid vs. monocytic lineage
Lymphoblastic Leukemia/Lymphoma (LBL)
Nodal or extranodal involvement is common at presentation
T-LBL may present with mediastinal mass
B-LBL is more frequently extranodal
Histologically there is diffuse and uniform infiltrate of small to intermediate-sized lymphoblasts
Immunophenotype of immature lymphoid cells; of B-cell more frequently than of T-cell lineage
Cytogenetic abnormalities are common and define subtypes
Chronic Myelogenous Leukemia, Blast Phase (CML-BP)
Nodal or extranodal myeloid blast proliferation occurs in ˜ 15% of cases of CML
Usually associated with blast phase in bone marrow or peripheral blood
Karyotype and FISH are required to establish
t(9;22)(q34;q11.2)
Complex cytogenetic abnormalities associated with blast phase
BCR-ABL fusion gene
RT-PCR can show BCR-ABL and quantify levels
Patients with t(8;22)(p11;q11) may present with leukocytosis and basophilia, simulating CML
Myeloproliferative Neoplasm (MPN) or Myelodysplastic/MPN (MDS/MPN)
MPN or MDS/MPN may be associated with lymphadenopathy
Lymph node involvement may be similar to lymphomas associated with FGFR1 abnormalities
Myeloid infiltrates may contain variable amounts of lymphoblasts, usually of T-cell lineage
Negative for cytogenetic or molecular features that define other diseases, e.g., BCR-ABL, JAK2, FIP1L1-PDGFRα
Further studies are required to define these processes
DIAGNOSTIC CHECKLIST
Clinically Relevant Pathologic Features
Lymphadenopathy associated with leukocytosis and eosinophilia should raise suspicion of this disease
Pathologic Interpretation Pearls
Lymphadenopathy with diffuse effacement due to lymphoblasts and myeloblasts
Bone marrow with features of MPN or MPN/MDS and eosinophilia
Peripheral blood may show leukocytosis and CML-like features
SELECTED REFERENCES
1. Jackson CC et al: 8p11 myeloproliferative syndrome: a review. Hum Pathol. 41(4):461-76, 2010
2. Tefferi A et al: Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 85(2):158-64, 2010
3. Vega F et al: t(8;13)-positive bilineal lymphomas: report of 6 cases. Am J Surg Pathol. 32(1):14-20, 2008
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree